J Clin Aesthet Dermatol. 2018;11(8):45–50
J Clin Aesthet Dermatol. 2018;11(8):45–50
by James Q. Del Rosso, DO; Leon Kircik, MD; and Emil Tanghetti, MD
Dr. Del Rosso is with JDR Dermatology Research and Thomas Dermatology in Las Vegas, Nevada, and is also Adjunct Clinical Professor at Touro University Nevada in Henderson, Nevada. Dr. Kircik is Clinical Associate Professor of Dermatology at Icahn School of Medicine at Mount Sinai in New York, New York, and is Medical Director at Indiana University Medical Center in Indianapolis, Indiana. Dr. Tanghetti is Medical Director at the Center for Dermatology and Laser Surgery in Sacramento, California.
Funding: This study was sponsored by Allergan plc (Dublin, Ireland).
Disclosures: Drs. Del Rosso and Kircik are advisors, investigators, speakers, and/or consultants for Allergan. Dr. Tanghetti is a consultant and/or investigator for Galderma, Allergan, Hologic, Novartis, and Ortho Derm.
Abstract: The majority of available data on the prevalence, grading, and management of acne vulgaris (AV) are based on studies that evaluate facial AV. Data are limited in all of these areas with truncal AV. This study evaluates the efficacy and safety of topical management of truncal AV involving the chest and back in a three-center, open-label, 16-week study. Enrolled subjects (N=20), 12 years of age or older, applied dapsone 7.5% gel once daily as monotherapy. The primary endpoint of the study was the percent of subjects who achieved a two-grade improvement and a rating of clear or almost clear based on the Investigator Global Assessment scale. Secondary endpoints included percent reductions of inflammatory, non-inflammatory (comedonal), and total lesions at Week 16 compared to baseline. Tolerability and safety were assessed over the duration of the study.
Keywords: Acne vulgaris, acne, truncal, topical, gel, dapsone
Acne vulgaris (AV) is the most common cutaneous disorder seen in ambulatory dermatology practice among the pediatric and adult populations.1 The vast majority of data on the epidemiology and management of AV are based on studies evaluating facial AV, with prevalence and treatment data far more limited for truncal AV.2–7 Studies completed over the past decade have reported that truncal AV (chest and/or back) affects approximately 50 to 60 percent of individuals who present with AV, with the majority of cases concurrent with facial AV; intra-individual differences in severity can be present among the facial and truncal sites affected by AV.4,5,8 Among over 2,000 adolescents studied, a high level of dissatisfaction with truncal AV was reported in approximately half of the 18-year-old male patients affected by AV on the chest and/or back.9 Additionally, as the majority of affected patients desire treatment for truncal AV, there is a definite need to develop and validate severity grading methods, efficacy parameters, and quality-of-life (QoL) assessments specific for truncal AV.4,7,10
Because of the more extensive body surface area involvement in truncal AV compared to the face, oral therapy is often perceived to be a necessary part of the treatment regimen.6,7 In cases of severe inflammatory and nodular AV, this is a reasonable consideration. Other than in cases warranting treatment with oral isotretinoin, combination therapy with a topical regimen is recommended, especially concomitant with oral antibiotic therapy in patients with truncal AV where use of an oral agent is deemed necessary.2,5–7,11 However, many cases of truncal AV can be mild to moderate in severity, and thus could potentially respond adequately to a topical regimen as initial treatment or as maintenance therapy after discontinuation of oral antibiotic therapy.6,7,12
This study assessed efficacy and safety of dapsone 7.5% topical gel in the management of truncal AV involving the chest and back when applied once daily as monotherapy in patients 12 years of age or older. Three centers enrolled subjects with truncal acne for evaluation in a 16-week, open-label, pilot study.
Clinical Study Protocol Overview
Study objectives and enrollment population. The objectives of this study were to observe and document the efficacy and safety of the brand formulation of topical dapsone 7.5% gel (Aczone gel 7.5%, Allergan Pharmaceuticals, Irvine, California, USA) as monotherapy for truncal AV. The study included 20 subjects at least 12 years of age with truncal AV involving the chest and back that was rated predominantly as moderate in severity. The study did not include subjects with predominantly nodular inflammatory lesions. Among our subjects, the predominant inflammatory lesions were papules and pustules, and non-inflammatory lesions included closed and open comedones. Eligible subjects included both sexes and all skin types. The study was completed in accordance with the Guideline for Good Clinical Practice (GCP), followed ethical principles described in the current revision of the Declaration of Helsinki, and was Investigational Review Board (IRB)-approved. All standards of drug shipment, storage, dispensing, and inventory were followed and monitored as specified in the IRB-approved protocol.
Inclusion and exclusion criteria and study withdrawal procedures. Inclusion criteria included the following:.
Outpatient subjects were male or female of any race, and at least 12 years of age. Female subjects of childbearing potential required a negative urine pregnancy test (UPT) result at baseline (sensitivity of at least 25mIU/mL for human chorionic gonadotropin) and were instructed to practice a reliable method of contraception throughout the study.
All subjects were required to understand the requirements of the study and sign Informed Consent/ Health Insurance Portability and Accountability Act of 1996 (HIPAA) Authorization forms after assurance of their understanding of the study. Written and informed consent of a parent or legal guardian was required for subjects under their state-mandated legal age of consent.
Entry into the study required that the subject was rated with at least a truncal acne severity score of 3 (moderate severity) at baseline.
Exclusion criteria included the following:
Female subjects who were pregnant (positive UPT), breastfeeding, or who were of childbearing potential and not practicing a reliable method of birth control
Individuals with allergies or sensitivity to any component of the test medication
Subjects who did not comply with the proper wash-out periods for prohibited medications were excluded (standard washout periods approved by IRB were incorporated).
Concurrent use of any topical or oral medications used to treat AV or pigmentary disorders (i.e., bleaching agents) or any physical therapies or modalities (i.e., lasers, lights, peels) during the course of the designated washout period and throughout the duration of the study
Presence of any medical condition that the investigator determined would contraindicate the subject from study participation, including any skin disorder that might interfere with the diagnosis or evaluation of AV.
Evidence of recent alcohol or drug abuse or exposure to an investigational drug study within 30 days of the baseline visit
History of poor cooperation, nonadherence to medical treatment, or unreliability.
Study withdrawal procedures. If it was determined that a subject’s health or well-being was threatened by continuation in the study, the subject would be withdrawn from the study. Circumstances that warranted study discontinuation included emergence of a serious adverse event (SAE), inability to physically or mentally tolerate use of the test medication, recognition of an exclusion criterion that became apparent at any time during the study, and the subject’s desire to voluntarily withdraw from the study at any time. In the event of premature discontinuation from the study, the primary reason for discontinuation was documented whenever possible.
Designated study visits. After completion of informed consent and other necessary forms for study entry, demographic information, vital signs, medical history, concomitant medications, physical exam, acne lesion counts, Investigator Global Assessment (IGA), skin tolerability assessments (i.e., erythema, dryness, peeling, oiliness, pruritus), adverse events (AEs), and UPT (when applicable) were obtained at all relevant visits as designated in the IRB-approved study protocol. An IRB-approved patient preference questionnaire was completed at the end of the study. The duration of the study was 16 weeks with visits scheduled at screening, baseline, and three follow up visits—Weeks 4, 10, and 16. Adherence by subjects to the study treatment regimen was assessed at each visit; missed applications of study medication were noted. Concomitant therapies not determined to interfere with the response to treatment could be continued at the discretion of the investigator. All concurrent medications (prescription or over-the-counter [OTC]) were recorded along with the reason the medication was taken.
Study medication application. Application sites to be treated during the study included the back and/or chest involved with AV of at least moderate severity. Affected skin was to be gently washed and patted dry, with approximately a pea-sized amount of dapsone 7.5% gel applied in a thin layer once daily. All subjects received the same brand of active study medication to be rubbed in gently and completely to the entire affected areas on the trunk.
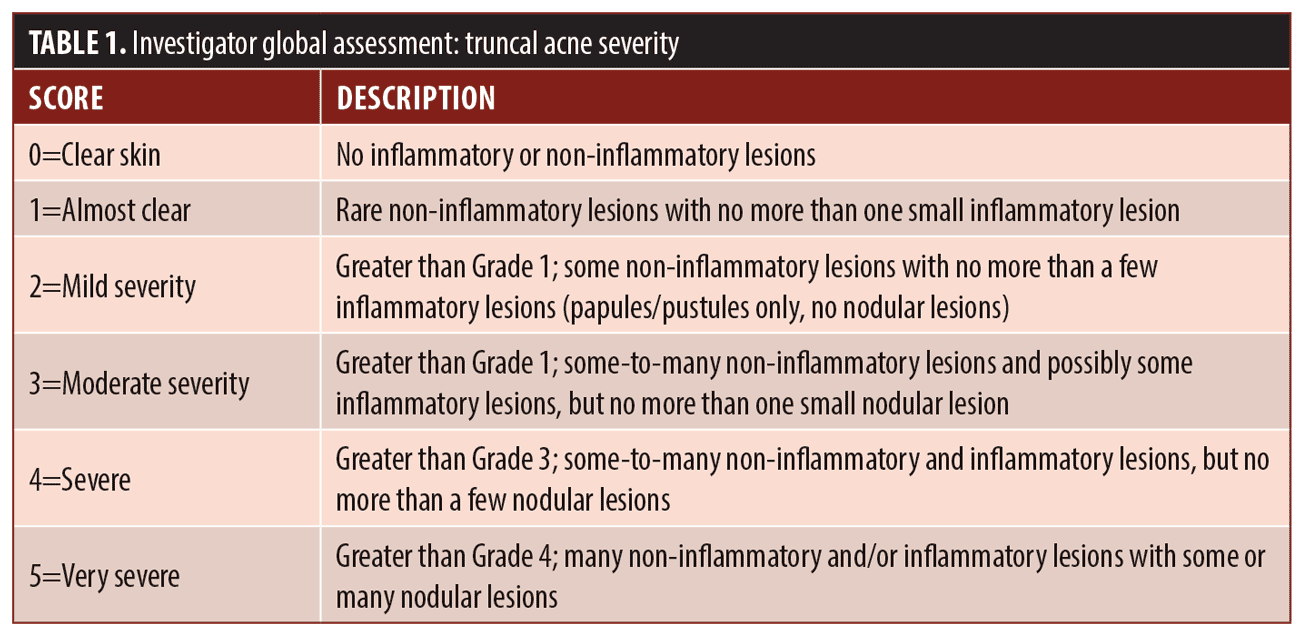
Efficacy assessments. Primary efficacy endpoints. The primary efficacy endpoints in this study were the percent of subjects who achieved at least a two-grade improvement and the percent of subjects who were rated as clear or almost clear at end of study compared to baseline according to the IGA scale for truncal acne. Evaluation of global truncal acne severity was completed using the IGA scale in Table 1.
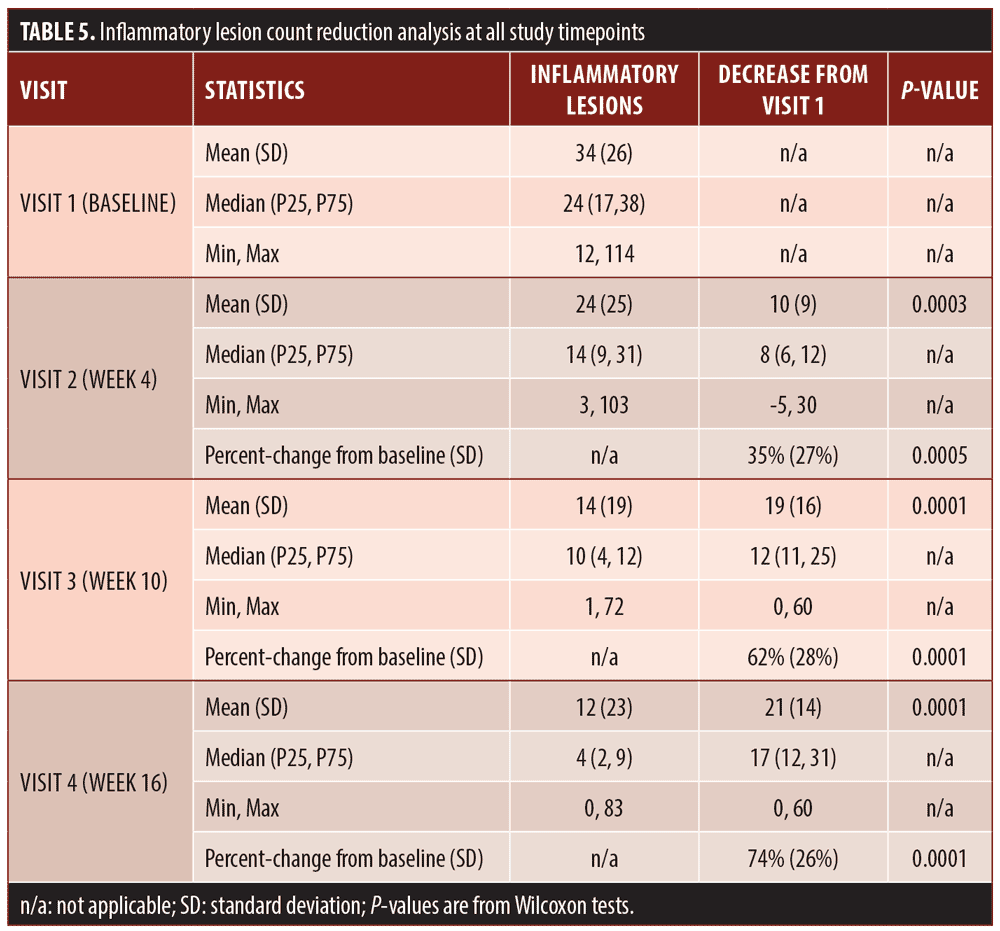
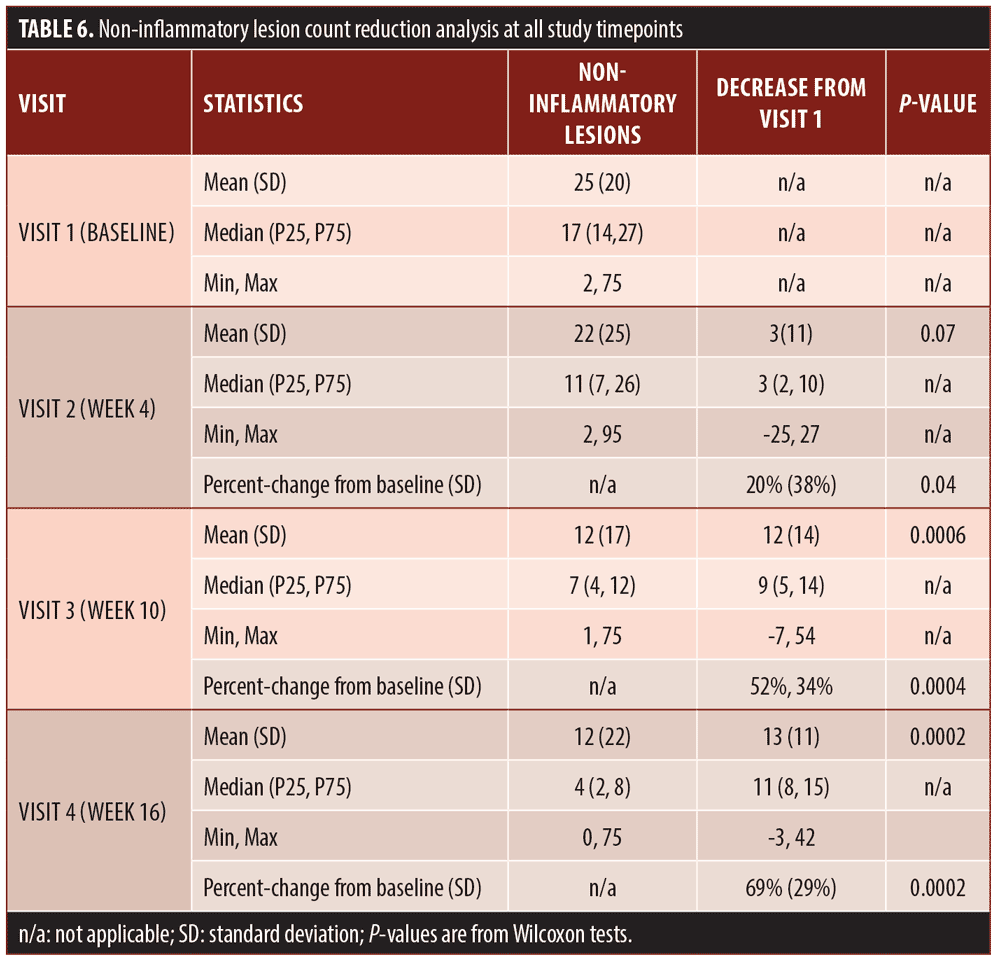
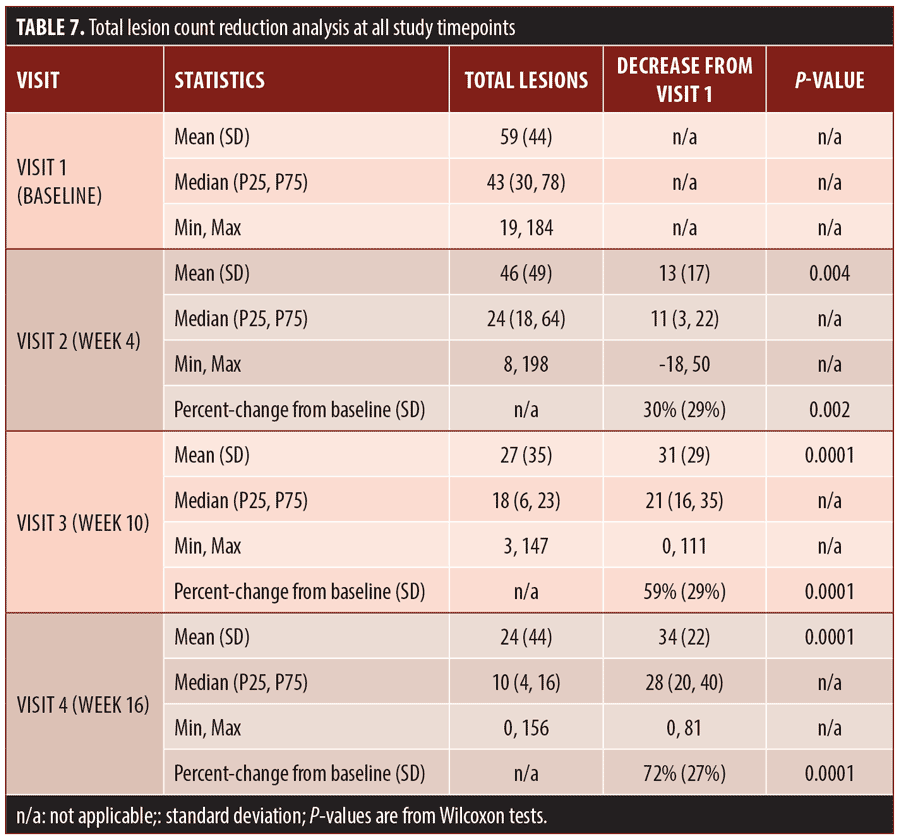
Secondary efficacy endpoints. The secondary efficacy endpoints evaluated were the percent reduction in inflammatory lesion count at Week 16 compared to baseline, percent reduction in non-inflammatory (comedonal) lesion count at Week 16 compared to baseline, and percent reduction in total lesion count at Week 16 compared to baseline.
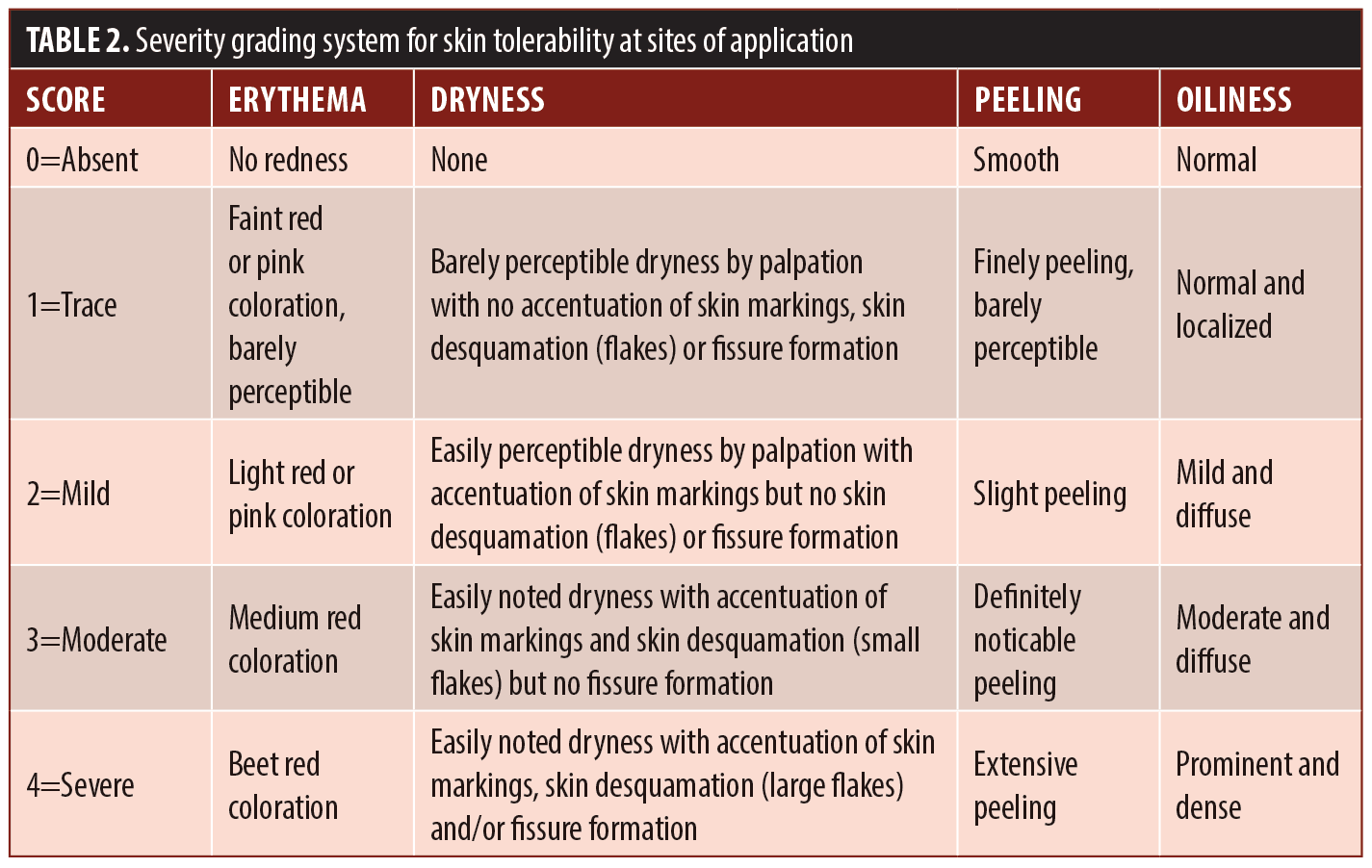
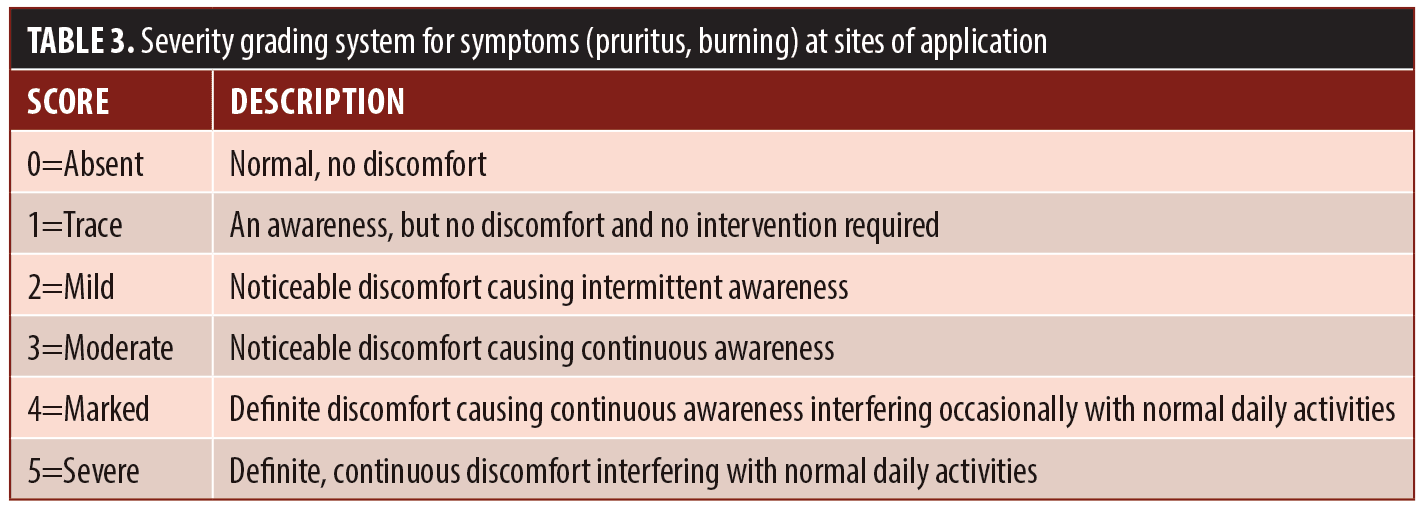
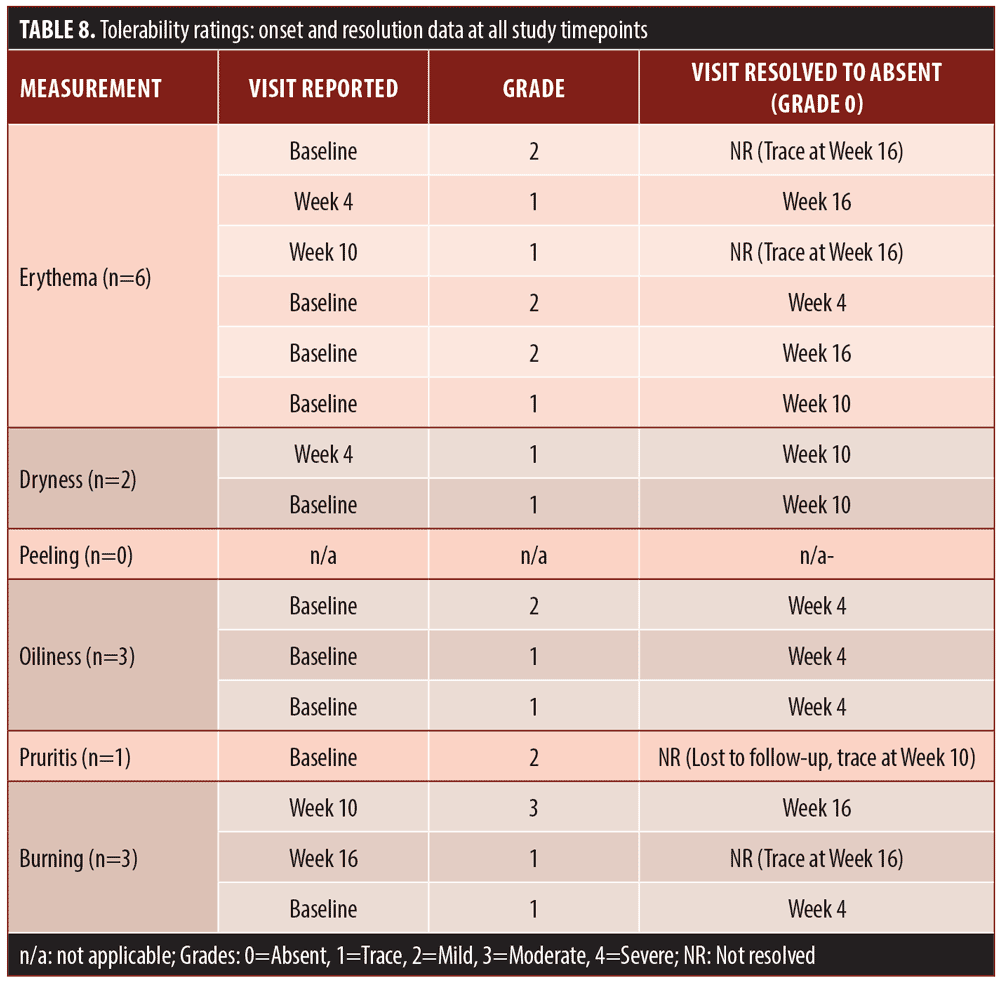
Tolerability assessments. At each visit the investigator graded current severity of erythema (both disease-related and/or study medication-related), dryness, peeling, and oiliness using the scale shown in Table 2. Current severity of symptoms of pruritus and burning within the study medication application areas were graded at each visit using the scale shown in Table 3.
Adverse event reporting. All AEs were documented over the entire course of the study. Information relevant to the AE, such as onset and stop date, severity, course of action taken, as well as any pertinent data necessary to allow for a complete evaluation of the AE, was recorded. Any SAEs were immediately assessed and reported to the safety monitor along with completion of an additional SAE report for full documentation.
Sample size and statistical analysis. Twenty subject were enrolled in the study. Since this was a pilot study, there was no formal justification for the sample size. Statistical analyses were conducted on an intent-to-treat basis (i.e., all enrolled subjects were to be included in the analyses). Statistical testing was two-sided and interpreted at a five-percent significance level. Descriptive statistics (i.e., mean, standard deviation) is provided for all continuous variables and frequencies for all categorical variables collected in this study. P-values were determined using Wilcoxon testing. The incidence and severity of individual AEs was tabulated.
Results
Subject and enrollment characteristics. As noted above, 20 subjects were enrolled in the study, with 15 completing the full duration. Five subjects were lost to follow-up—one after the first visit (Week 0) and four after the third visit (Week 10). Efficacy and tolerability results are shown for all 20 enrolled subjects, including the five lost to follow-up, using missing values imputed by last observation carried forward (LOCF). The patient preference questionnaire was completed by the 15 subjects who completed the full study.
The average age of enrolled subjects was 21 years with an age range of 12 to 41 years. The ethnicity mix was 70 percent (n=14) white, 10 percent (n=2) non-Hispanic, 10 percent (n=2) biracial, five percent (n=1) Puerto Rican, and five percent (n=1) “White Dominican.”
Efficacy outcomes. Baseline severity ratings and acne lesion counts are shown in Tables 4 to 7. Sixteen subjects (80%) were rated at baseline as moderate in acne severity, and the remaining four subjects (20%) were rated as severe at baseline. The percentage of subjects achieving a two-grade improvement by Week 16 was 55 percent, and the percentage of subjects rated as clear or almost clear by Week 16 was 45 percent (Table 4). Reductions in inflammatory lesion counts (Table 5), non-inflammatory (comedonal) lesion counts (Table 6), and total lesion counts (Table 7) decreased progressively over the duration of the study, with percent change from baseline statistically significant at all time points. By Week 16, inflammatory lesions, non-inflammatory (comedonal) lesions, and total lesions decreased by 74 percent (p=0.0001), 69 percent (p=0.0002), and 72 percent (p=0.0001), respectively, compared to baseline.
Tolerability assessments. There were fifteen reports of erythema, dryness, oiliness, pruritus, or burning (Table 8). Ten of these reports were rated as trace or mild and resolved to absent by the end of study, one was rated as moderate and resolved to absent by the end of study, three were rated as trace and were unresolved at the end of the study, and one was trace at Week 10 and was lost to follow-up.
AE outcomes. There was a total of four AEs reported in three subjects. Three of the AEs were determined by the investigator to be definitely unrelated to study medication (leg abrasions with infection, oral surgery, sty), and one determined to be unlikely related to study medication (sunburn).
Patient preference questionnaire overall results. Among vehicles that study subjects had previously used, gel was the most preferred, followed closely by the lotion and cream. Ointment and spray were generally not preferred, but also had been used by fewer study subjects in the past. Overall, most subjects preferred the study medication over previously used medications, and most subjects commonly rated its qualities as good or excellent (i.e., easy to apply to large area, absence of smell, lack of stickiness/greasiness).
Summary
Management of truncal AV has received limited emphasis over the years, especially compared to the plethora of studies completed on therapies for facial AV. However, truncal AV affects at least half of all individuals affected by facial AV, with many expressing interest for treatment of AV involving their trunk.4–9 Nevertheless, there is a conspicuous absence of many randomized, controlled trials (RCT) evaluating medical management of truncal AV.6,7,12 Therapies that have been evaluated more recently for truncal AV have included azelaic acid 15% foam, benzoyl peroxide foam including short contact therapy, combination oral contraceptive treatment (drosperinone 3mg/ethinyl estradiol 0.02mg), and photodynamic therapy (5% 5-amonolevulinic acid).12–16
This pilot 16-week study (N=20) evaluated monotherapy using once daily topical application of dapsone 7.5% gel for AV involving the back and/or chest. The majority of subjects (80%) presented at baseline with moderately severe truncal AV; the remainder exhibited severe truncal AV. Study outcomes showed that topical dapsone 7.5% gel was well-tolerated and well-accepted by study subjects. Efficacy was demonstrated both by IGA assessments and acne lesion counts. By the end of the study (Week 16), the percentage of subjects achieving a two-grade improvement in IGA rating and the percentage of subjects graded as clear or almost clear were 55 and 45 percent, respectively. Over the duration of the study, all acne lesion counts exhibited a marked progressive reduction, with inflammatory lesions, non-inflammatory (comedonal) lesions, and total lesions decreasing by 74, 69, and 72 percent, respectively, at the end of the study compared to baseline. The results of this pilot study suggest that dapsone 7.5% gel is a viable option to add to the armamentarium for treatment of truncal AV. A large-scale RCT evaluating this agent for truncal AV would be helpful in further defining its efficacy in this population of patients with AV.
References
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11(4):
466–473. - Thiboutot D, Gollnick H, Bettolli V, et al. New insights into the management of acne: an update from the global alliance to improve outcomes in acne group. J Am Acad Dermatol. 2009;60:s1–50.
- Del Rosso JQ. Topical and oral antibiotics for acne vulgaris. Semin Cutan Med Surg. 2016;35(2)
:57–61. - Del Rosso, JQ, Bikowski JB, Baum E, et al. A closer look at truncal acne vulgaris: prevalence, severity, and clinical significance. J Drugs Dermatol. 2007;6(6): 597–600.
- Tan JK, Tang J, Fung K, et al. Prevalence and severity of facial and truncal acne in a referral cohort. J Drugs Dermatol. 2008;7(6):551–556.
- Del Rosso JQ. Truncal acne vulgaris: the relative roles of topical and systemic antibiotic therapy. J Drugs Dermatol. 2007;6(2):148–151.
- Del Rosso JQ. Management of truncal acne vulgaris: current perspectives on treatment. Cutis. 2006;77(5): 285–289.
- Dreno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Euro Dermatol Venereol. 2015;29(6):1096–1106.
- Isaacsson VC, Almeida HL Jr, Duqula RP, et al. Dissatisfaction and acne vulgaris in male adolescents and associated factors. An Bras Dermatol. 2014;89(4):
576–579. - McClellan C, Frey MP, Tan J. Assessing the need for a comprehensive acne quality-of-life scale for face and torso acne. J Cut Med Surg. 2018;22(3):262–266.
- Layton AM. Top ten list of clinical pearls in the treatment of acne vulgaris. Dermatol Clin. 2016;34:147–157.
- Hoffman LK, Del Rosso JQ, Kircik LK. The efficacy and safety of azelaic acid 15% foam in the treatment of truncal acne vulgaris. J Drugs Dermatol. 2017;16(6):5
34–538. - Bikowski J. A review of the safety and efficacy of benzoyl peroxide (5.3%) emollient foam in the management of truncal acne vulgaris. J Clin Aesthet Dermatol. 2010;3(11):26–29.
- Leyden JJ, Del Rosso JQ. Effect of benzoyl peroxide 9.8% emollient foam on reduction of Propionibacterium acnes on the back using a short contact therapy approach. J Drugs Dermatol. 2012;11(7):830–833.
- Palli MB, Reyes-Habito CM, Lima XT, et al. A single-center, randomized double-blind, parallel group study to evaluate the safety and efficacy of 3mg drosperinone/0.02mg ethinyl estradiol compared with placebo in the treatment of moderate truncal acne vulgaris. J Drugs Dermatol. 2013;12(6):633–637.
- Yew YW, Lai YC, Lim YL, et al. Photodynamic therapy with topical 5% 5-aminolevulinic acid for the treatment of truncal acne in Asian patients. J Drugs Dermatol. 2016;15(6):727–732.