 J Clin Aesthet Dermatol. 2020;13(8):28–35
J Clin Aesthet Dermatol. 2020;13(8):28–35
by Mao-ying Lin, MD; Chrang-shi Lin, MD; Sindy Hu, MD; and Wen-hung Chung, MD, PhD
Dr. MY Lin is with the Department of Dermatology at Xiamen Chang Gung Hospital in Xiamen, Fujian, China. Dr. CS Lin is with National Yang-Ming University and Dr. Lin Skin Clinic in Taipei, Taiwan. Dr. Hu is with the Department of Dermatology at Xiamen Chang Gung Hospital in Xiamen, Fujian, China and the Department of Dermatology at Chang Gung Memorial Hospital in Taoyuan, Taiwan. Dr. Chung is with the Department of Dermatology at Xiamen Chang Gung Hospital in Xiamen, Fujian, China and the Department of Dermatology at Chang Gung Memorial Hospital in Linkou, Taiwan.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Platelet-rich plasma (PRP) has been receiving considerable attention in the field of dermatology since the elucidation of its mechanism and reports of its clinical efficacy. PRP alone or in combination with other therapies has demonstrated benefits for some cosmetic problems and skin diseases. Only a few transient or short-term side effects have been reported with the use of PRP. In this review, we highlight the potential efficacy and benefits of PRP with a focus on its applications in skin rejuvenation, androgenic alopecia, alopecia areata, chronic vitiligo, melasma, inflammatory nail disorders, and psoriasis. We suggest that detailed studies be conducted to standardize PRP preparation and optimize treatment methods in order to further improve its usefulness.
Keywords: Platelet-rich plasma, dermatology, cosmetics, medical
Platelet-rich plasma (PRP), a biological product, is a portion of the plasma fraction of autologous blood with platelet concentration above the baseline. PRP not only contains a high level of platelets, but also has a full range of clotting factors. Moreover, it is enriched with several growth factors (GFs), chemokines, cytokines, and other plasma proteins.1
In the preparation of PRP, anticoagulants, such as acid citrate dextrose or sodium citrate, are added to whole blood (10–60mL) to prevent ex-vivo coagulation and premature secretion of alpha granules. The anticoagulated blood is subsequently subjected to a soft spin (i.e., slower speed ± shorter duration) to obtain three layers: a base layer of red blood cells, a central buffy coat layer enriched in white blood cells, and a top plasma layer enriched in platelets while also containing few white blood cells. Different layers of the product are transferred to a fresh tube when pure PRP (P-PRP) and leukocyte-rich PRP (L-PRP) are desired, and these are separately subjected to a hard spin (i.e., higher speed ± longer duration). When P-PRP is desired, only the most superficial buffy coat with the lower plasma layer is collected and centrifuged; conversely, when L-PRP is desired, the entire buffy coat with the lower plasma layer is collected and centrifuged. Calcium gluconate, calcium chloride, or thrombin can be added to activate GF secretions before the administration of PRP (Figure 1).1–4
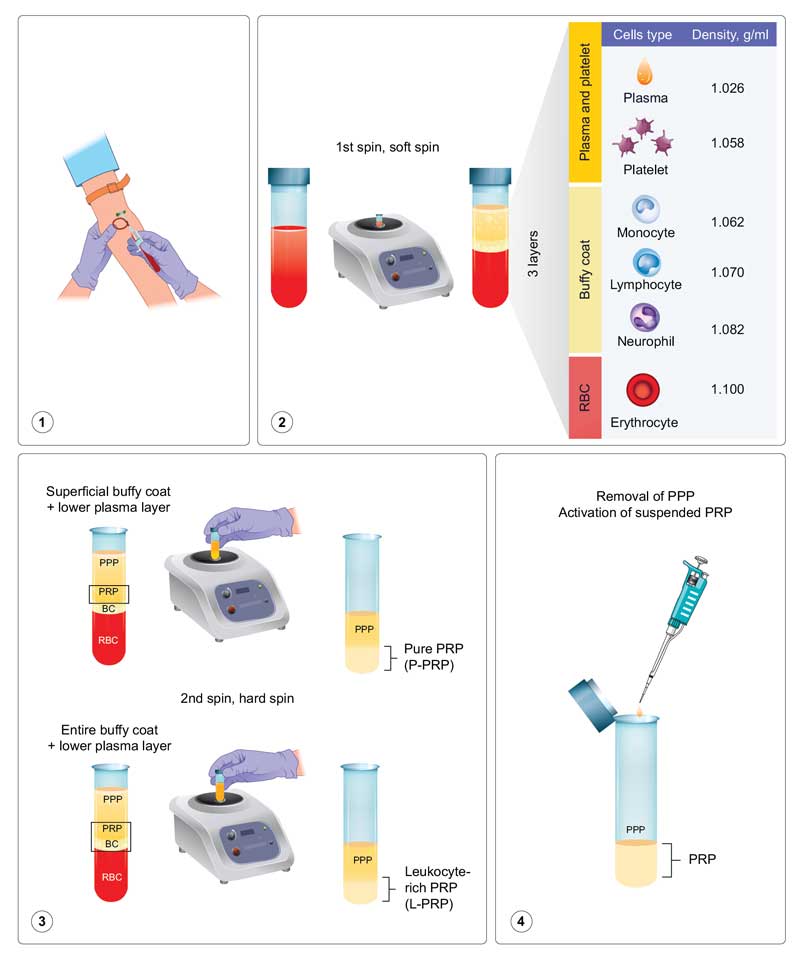
Nonactivated PRP makes use of host dermal collagen and thrombin as endogenous activators. The highest platelet capture efficiency with preserved platelet functions of P-PRP has been reported using the following parameters: 160 × g for 10 minutes in the first spin and 250 × g for 15 minutes in the second spin.2,3 There are more than 20 native GFs in PRP, including platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-?), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and insulin-like growth factor 1 (IGF-1). Various types of GFs bind to cell surface receptors and activate cell signaling pathways, resulting in the expression of genes and the synthesis of various proteins required for mitogenesis, for increasing cell numbers, and angiogenesis, for stimulating vascular growth. PRP is generally defined as plasma containing 1,000,000 platelets/?L. Because an individual’s baseline platelet count can vary, there can be inconsistencies in quantifying the “fold” increase. Therefore, the absolute number of platelets in PRP and the individual’s own platelet count on the day of treatment should be determined and considered.4,5 In addition to baseline platelet count, several other variables play a part in preparing the most efficient PRP. There is an intensive ongoing debate regarding these variables, including the terminology used for description, classifications (e.g., four-category, PAW, and DEPA classification), various techniques and preparation systems for PRP (e.g., activators; number, speed, and time of centrifugations; temperature; test tube materials), ideal volume, and technique, site, and frequency of administration.4,6,7 We advocate for the four-category classification (P-PRP, L-PRP, P-PRF, and L-PRF; based on leukocyte and fibrin contents) proposed by Dohan et al6 for simplified clinical application.
PRP has recently emerged as a treatment choice for a variety of dermatological conditions and concerns. This review aims to highlight the potential efficacy and benefits of PRP in skin rejuvenation, androgenic alopecia (AGA), alopecia areata (AA), chronic vitiligo, melasma, inflammatory nail disorders, and psoriasis.
Skin rejuvenation
Skin aging results in wrinkles, coarseness, pigmentation, and loose skin. PRP can induce remodeling of the extracellular matrix (ECM). This increases the expression of matrix metalloproteinases to remove photodamaged ECM components and stimulates the proliferation of dermal fibroblast and synthesis of collagen. However, the PRP-mediated skin rejuvenation is not dose-dependent. Recently, 5% PRP was reported to more strongly induce the production of procollagen Type I carboxy-terminal peptide than 10% PRP.8 A similar result was observed in fibroblast proliferation assay in vitro, with 5% PRP inducing higher fibroblast proliferation than 10% PRP.8 Activin A (ActA) is a member of the TGF-? superfamily. Administration of PRP in irradiated rats exposed to a radiation-delivering cobalt-60 source produced strong ActA/follistatin (FST) signaling on Day 7, parallel with significantly pronounced inflammatory response.9 On Day 28 after irradiation, ActA/FST signaling was inhibited and the inflammatory response was suppressed, whereas Collagen I expression was highly activated. Histological evaluation results indicated that the morphology was better in the PRP-treated irradiated group on Day 7 than in the non-PRP-treated irradiated group and the epidermal and dermal layers showed organized morphology similar to that in the nonirradiated group on Day 28. To verify the efficacy of PRP rejuvenation on human skin, Abuaf et al10 performed punch biopsies in the infra-auricular area at three time points: before injection, 28 days after PRP injection (RegenLab® kit; Regen Lab SA, Le Mont-sur-Lausanne, Switzerland), and 28 days after saline injection. Collagen fiber bundles in the dermis and the number and thickness of elastic fibers were significantly increased relative to baseline in groups treated with PRP and saline. Furthermore, the density of collagen fibers was significantly higher in the PRP-treated group than in the saline-treated group. The authors concluded that PRP increased dermal collagen levels not only through GFs but also via skin needling (the mesotherapy technique). Moreover, they suggested PRP application to be a safe and effective procedure, even after a single application, for facial skin rejuvenation.10
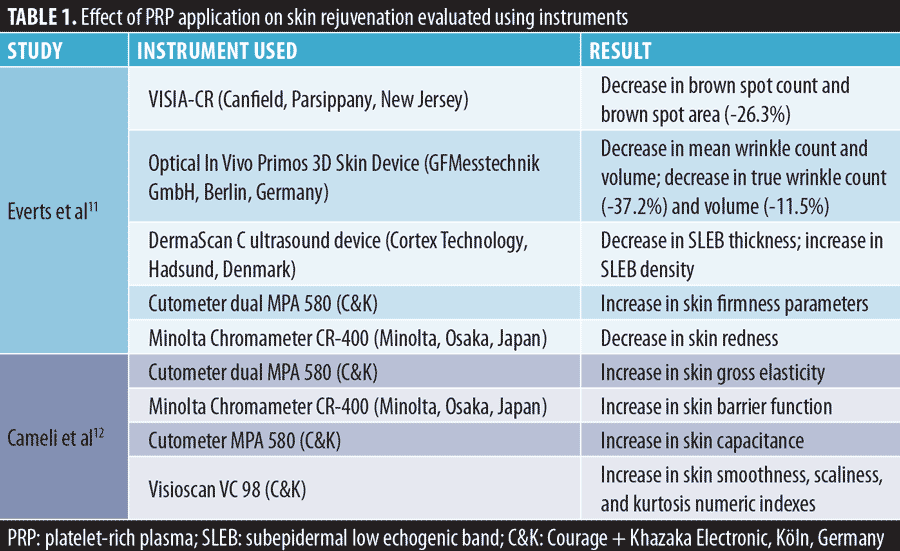
Objective assessments of PRP have also demonstrated efficacy for skin rejuvenation. Everts et al11 and Cameli et al12 administered three regimens of PRP injection at one-month intervals; the results of their studies are presented in Table 1.
Although PRP has been used clinically for skin rejuvenation for several years, the first randomized controlled clinical trial was conducted in 2018 by Alam et al.13 PRP and saline were injected in the same subjects on one cheek and the contralateral cheek, respectively. At six-months’ follow-up, masked participants rated the conditions and found that the PRP-treated cheek showed significant improvement in skin texture and wrinkles compared to the saline-treated cheek.13 Elnehrawy et al14 reported that, after a single PRP injection, nasolabial folds responded most favorably to treatment, followed by crow’s feet and the transverse forehead lines. There was a significant improvement in fine wrinkles, skin homogeneity, and texture, with notable improvement in the fourth week and the greatest improvement observed in the eighth week after injection. Subjects younger than 40 years of age showed more pronounced improvements in wrinkle appearance than older subjects.14
Lee et al15 also administered a single PRP injection on the cheeks and reported that patients were significantly satisfied with the overall facial and cheek appearance based on FACE-Q appearance appraisal scale evaluation. On the FACE-Q postcare scales, the majority of patients reported being pleased with the result (74.2%) and agreed that “it [was] worth the time and effort” (80.0%). However, the majority also disagreed with that the results were “miraculous” (86.7%) or “fantastic” (70.0%). When the results were evaluated according to the Global Aesthetic Improvement Scale scores, 14 patients had aesthetic improvement and 17 patients experienced no change.
In a split-face, double-blind study, PRP and ready-made GFs (MRS FACE, MRS Lift solution; Mesologica, Jakarta Barat, Indonesia) were used for skin rejuvenation every two weeks for a period of three months.16 Until one month after the last administration, no significant differences in the final epidermal and dermal thicknesses were observed between both groups. However, at six months after the last administration, the PRP group showed sustained improvement, whereas the group given ready-made GFs showed a decrease in both epidermal and dermal thickness. The authors concluded that the PRP group showed sustained effects because GFs and cytokines were naturally induced by PRP, whereas the ready-made GFs applied directly by mesotherapy had shorter life.16
Sclafani et al17 used platelet-rich fibrin (PRF) to correct deep nasolabial folds. Immediately after treatment, the average wrinkle assessment scale score was reduced by 2.17±0.56 points. One week after treatment, the reduction was 0.65±0.68 points, which increased to 0.97±0.75, 1.08±0.59, and 1.13±0.72 points at two, six, and 12 weeks after treatment, respectively. The authors suggested that PRF could provide long-term diminution of deep nasolabial folds without causing excessive fibrosis, which can result by using foreign materials. In addition, PRF showed a significantly greater ability to induce collagen matrix synthesis, cell proliferation, fibroblast migration, and messenger RNA expression of TGF-?, collagen 1, and fibronectin relative to PRP.18
Based on the evidence in the literature, PRP appears to be effective and safe for skin rejuvenation. The adverse effects of PRP, such as burning sensation, erythema, swelling, and ecchymosis, are transient and resolve in less than two weeks. To our knowledge, only one randomized controlled trial (RCT) has been reported. Further RCTs are required to determine the number of optimal sessions, frequency of application, ideal volume, and technique of administering PRP for skin rejuvenation.
PRP for Alopecia
The mechanism of PRP treatment in relation to hair folliculogenesis and hair cycling has only been partially elucidated. GFs bind to the tyrosine kinase receptors on dermal papilla cells to activate the MAPK/ERK pathway, leading to proliferation, differentiation, and cellular survival; at the same time, these can also activate AKT and Bcl-2 and decrease BAD/BAX, resulting in an antiapoptotic effect.19 GFs, such as VEGF, EGF, hepatocyte growth factor, fibroblast growth factor, and IGF-1, have angiogenic potential and can increase vascular structures around hair follicles.1 After reviewing 19 articles published in 2019, Hesseler et al3 concluded that PRP is beneficial for hair growth. To reduce injection pain and minimize subjective bias, such as different depths and volumes, automatic injectors could be an alternative to manual administration.20 Furthermore, topical anesthesia with EMLA (AstraZeneca, Cambridge, United Kingdom), an air-cooling device (Zimmer Biomet, Warsaw, Indiana), and vibratory distracting devices could be helpful for pain relief. However, local anesthesia should be used with caution because it affects the pH of PRP and has been reported to have a negative impact on efficacy.4
Androgenic alopecia. The effect of PRP on the yield of follicular units in male baldness surgery was observed for the first time by Uebel et al21 in 2006; they reported a significant improvement over conventional techniques. We have reviewed six RCTs on the efficacy of PRP injection for AGA treatment in this study (Table 2).
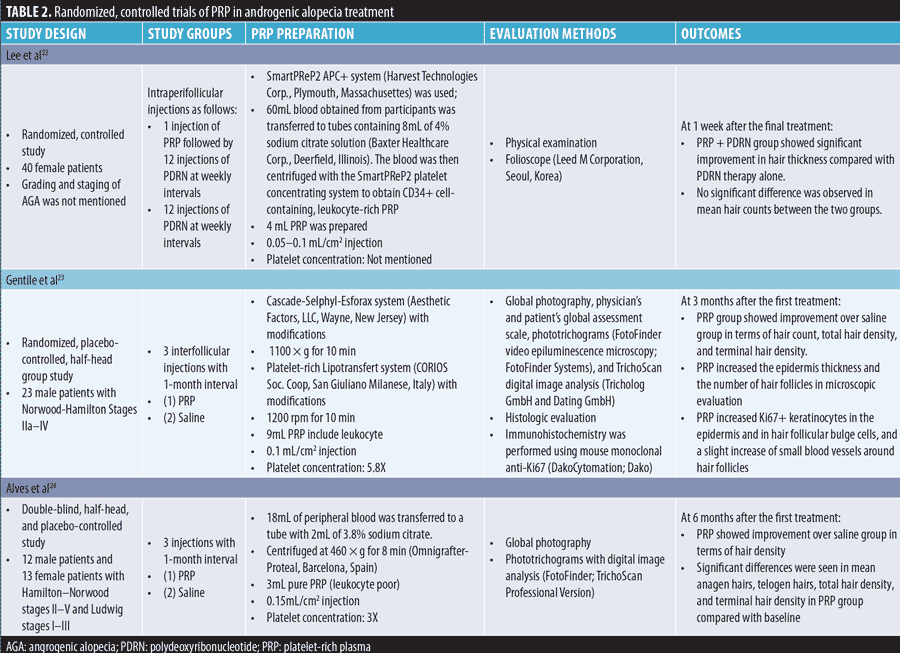
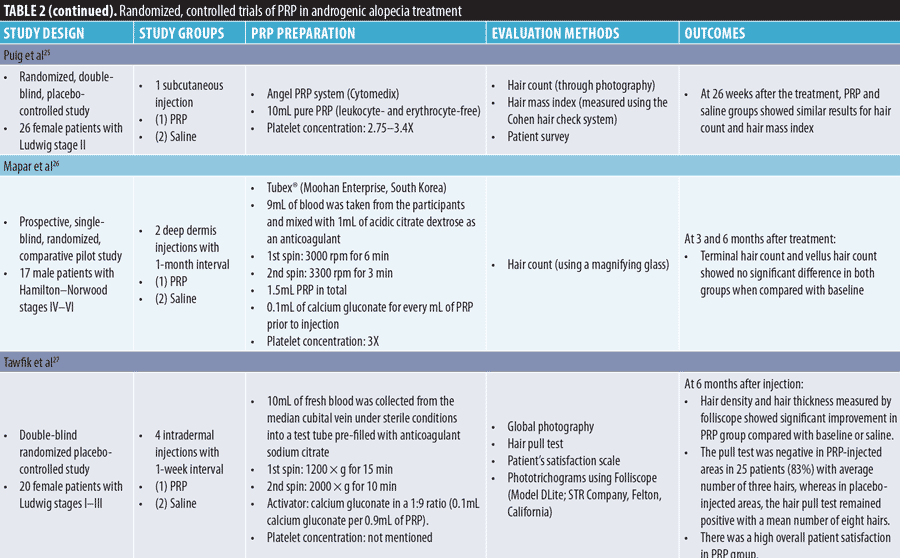
Several advanced PRP products have been introduced for AGA treatment. Kang et al28 compared the efficacy of interfollicular injections of PRP preparation containing CD34+ cells (SmartPReP®2 APC+ and SmartPReP®2 platelet concentrating systems; Terumo BCT, Lakewood, Colorado) administered twice at three-month intervals with that of placental extract injections (Melsmon®, Tokyo, Japan) administered at one-week intervals for six months. CD34+ cell-containing PRP treatment showed a significantly higher degree of improvement in hair thickness and overall clinical improvement than placental extract treatment; this was not observed for hair count. The therapeutic use of autologous CD34+ hematopoietic stem cells has been demonstrated to safely promote angiogenesis and vasculogenesis under ischemic conditions. The authors concluded that interfollicular injection of concentrated mobilized CD34+ cells in PRP preparations could have synergistic effects in patients with AGA.
Cole et al29 reported that transplanted grafts resumed growth faster in the presence of platelet lysate (PL; Arthrex Angel® system from Arthrex, Naples, Florida; and Bioruptor Plus® sonication device from Diagenode, Denville, New Jersey) than in the presence of a-PRP (Regen® Blood Cell Therapy; Regen Lab SA, Le Mont-sur-Lausanne, Switzerland) or saline in male patients with AGA. When PL was injected alone, the patients experienced a 50-percent increase in follicular unit density and 122-percent increase in hair density at seven months after injection. PL generated from a 30-minute sonication of PRP was found to have significantly higher (6- to 8-fold) levels of VEGF, PDGF-BB, and TGF-?1 than a-PRP, which explained the superior follicular regeneration rate of PL-treated grafts.
Ince et al30 compared the efficacy of nonactivated autologous PRP (n-PRP; Young Cell PRP kit), activated autologous PRP (a-PRP; Truecell PRP kit), and homologous PRP (h-PRP, pooled platelets prepared at the blood center) on AGA therapy. They found that h-PRP was most effective for AGA, followed by n-PRP and a-PRP; however, the reason for this difference was not fully understood. h-PRP had a higher platelet count than autologous PRPs. Autologous PRP has only one individual GF, whereas h-PRP has at least 4 to 5 donor GFs. Low levels of IGF-1 and VEGF after activation in the kit that the authors used might be responsible for the reduced efficacy of a-PRP therapy. Lastly, n-PRP possibly has a more balanced level of GFs.
Stevens et al31 reported the positive effect of booster injections with PRP and an adipose-derived stromal vascular fraction, or “platelet-rich stroma,” in male patients with AGA. Hair density significantly increased at six and 12 weeks after injection. Hair-to-hair matching analyses showed that new hair grew from active follicles. Nonfunctioning hair follicles filled with hyperkeratotic plugs also showed new hair growth. Adipose tissue-derived stem cells (ASCs) enable an increase in the proliferation of dermal papilla cells and activation of the anagen phase in hair cycles. This stimulates follicle regrowth and modulation of the hair cycle, as well as the production and secretion of GFs. The expression of these GFs allows ASCs to have an angiogenic capacity and the ability to induce tissue neovascularization and a microenvironment with an abundant blood supply for hair cells to regenerate hair follicles. ASCs also exert immunomodulatory and/or immunosuppressive effects via direct cell-to-cell interaction or secreted cytokines.
Thus, irrespective of whether PRP is administered alone or in combination with other agents, it seems to be a promising treatment for AGA. It should be noted that two RCTs conducted by Mapar et al26 and Puig et al32 showed no positive effect of PRP on AGA. Possible causes for the unfavorable results in these two studies include the following: (1) advanced stage of AGA; (2) low number of PRP injections (one injection in Puig et al and two injections Maper et al); (3) injections were too deep (subcutaneous in Puig et al and deep dermis in Maper et al); and (4) use of a subjective evaluation method only, whereas other studies used a folliscope as an objective evaluation method.
Alopecia areata (AA). Our review of published clinical trials revealed that PRP administered either alone or in combination with triamcinolone acetonide (TrA) effectively improved AA. The data of studies using PRP for AA treatment is presented in Table 3.
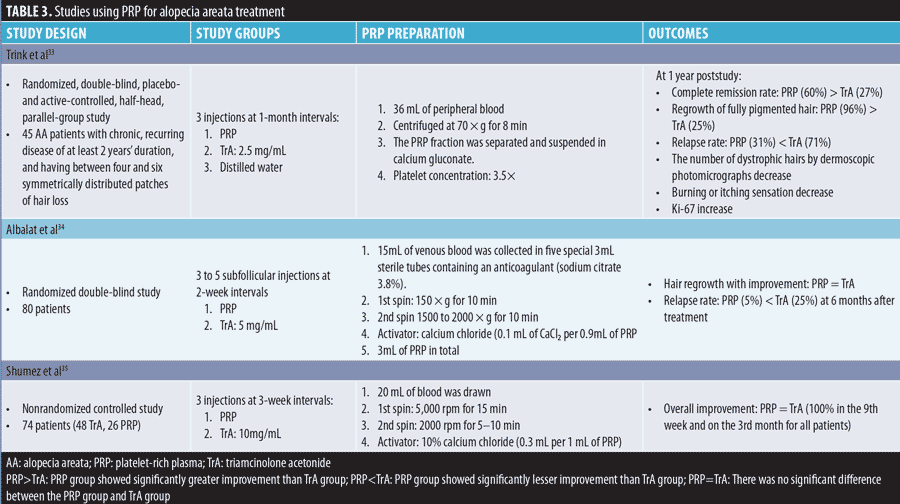
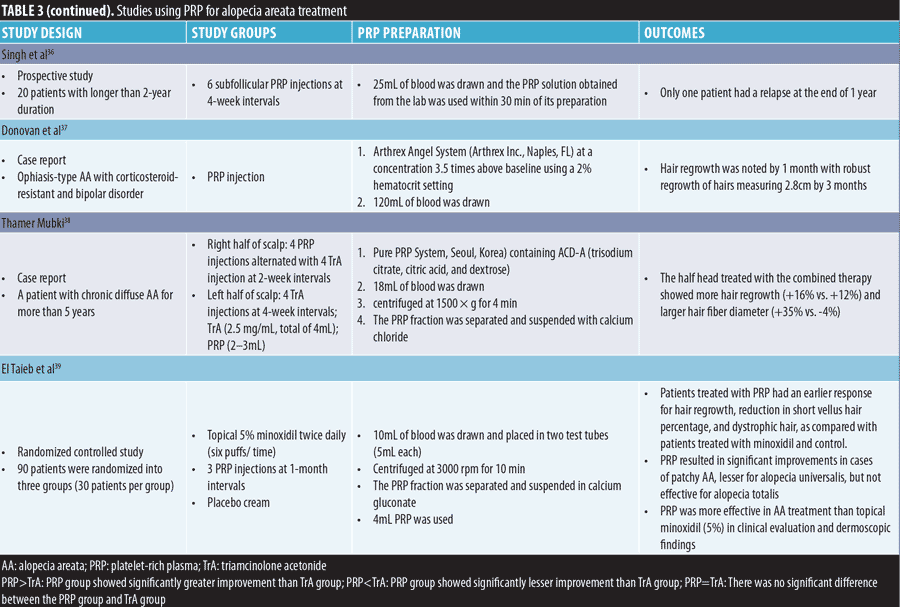
AA is considered an organ-specific autoimmune disease and affects self-esteem; it results in the loss of the immune privilege of hair follicles. Conventional therapies are mostly immunosuppressive in nature and have significant relapse rates and side effects. For instance, relapse rates of 29 percent and 72 percent were reported for intralesional corticosteroids in limited AA and alopecia totalis, respectively; moreover, relapse rates of 37 percent to 63 percent, 25 percent to 41 percent, more than 50 percent, and 100 percent were reported for topical steroids, oral steroids, methotrexate, and cyclosporine, respectively.36 For chronic AA, the relapse rate was significantly lower after intralesional PRP injections (5%–31%) than after intralesional TrA injections (25%–71%). The improvement in chronic AA showed no significant difference between PRP and TrA in two studies, but PRP showed significantly better results than TrA in one study. Mood changes associated with systemic corticosteroid administration are not uncommon. A high prevalence of mood disorders, such as major depressive disorder and anxiety, was observed in patients with AA.37 The mechanism of PRP in AA treatment remains unclear, but is likely the result of combination effects of proliferation and differentiation, anti-inflammatory effects, and immunomodulatory mechanisms induced by GFs.33 Apart from pain, no other side effects of PRP were reported in AA treatment. Further RCTs are required to validate the efficacy of PRP for AA by conducting long-term follow-up and evaluating the relapse rate and benefits of PRP for those who experience mood changes with steroids.
Cicatricial alopecia. Two biopsy-proven cases of primary scarring alopecia caused by cicatricial alopecia and lichen planopilaris were reported.40 Both cases showed no response to conventional therapies, but responded well after three injections of PRP administered at four-week intervals. Six months after treatment, hair loss resumed in both patients, indicating the need for maintenance therapy. For cicatricial alopecia, anti-inflammatory effects of GFs, as well as their ability to remodel collagen and the ECM, might contribute to their potential success. Although the results of this limited case series are encouraging, further studies are required to understand the possible role of this treatment.
Pigmentary disorders
Vitiligo. PRP treatment can improve vitiligo by several possible mechanisms:
- Growth factors: the stimulation of melanocyte regeneration is very important for vitiligo treatment. Basic fibroblast growth factor (bFGF) in PRP can significantly enhance the migration of melanocytes and the proliferation of keratinocytes and fibroblasts. Stem cell factor and bFGF are also essential for cell survival. Serum TGF-? levels are reduced in patients with nonsegmental vitiligo. Parambath et al41 proposed that TGF-? present in PRP leads to better response for stable vitiligo. Moreover, PRP could stimulate the undifferentiated stem cells.
- Anti-inflammatory effect: PRP exhibits anti-inflammatory effects and can suppress the release of cytokines, such as interleukin-1, interferon-c, and tumor necrosis factor-?. This, in turn, benefits the interaction between melanocytes and keratinocytes.41–46
- Matrix proteins: PRP containing fibrin, fibronectin, and vitronectin enhances cell adhesions between keratinocytes, fibroblasts, and melanocytes.46
In a double-blind RCT, stable vitiligo was treated with autologous transplantation of noncultured epidermal cell suspension (NECS). The results indicated that NECS in PRP resulted in significantly greater mean repigmentation and patient satisfaction compared to that when using phosphate-buffered saline.41 Mahajan et al42 showed that treatment with intralesional PRP injections, consisting of six injections at two-week intervals, was an effective therapy for chronic localized vitiligo patients who did not respond to traditional therapies. As for the treatment of stable nonsegmental vitiligo lesions, the combination of fractional CO? laser with intralesional PRP injection resulted in superior repigmentation to either the combination of fractional CO? laser with narrowband ultraviolet B (NB-UVB), or fractional CO? laser alone, or PRP injection alone.45,46 Another study also revealed that the combination of intralesional PRP injection with NB-UVB led to better repigmentation than NB-UVB alone.47 Vitiligo stability is crucial for the planning of treatment strategy. Ejjiyar et al43 reported the development of facial vitiligo in a female patient after PRP injections (Koebner phenomenon).
The desired content and possible role of leukocytes in PRP products have not been elucidated in previous studies. Against the presence of leukocytes in PRP, some research groups have advocated that it might have negative therapeutic outcomes due to the potential risk of stimulating the inflammatory process.6 In contrast, other groups insist that some leukocytes are required to increase GF production.6 Moreover, because the pathogenesis of vitiligo involves autoimmune-mediated destruction of melanocytes, the inhibition of autoimmunity could be considered as a vitiligo treatment, and P-PRP or L-PRP can be used by dermatologists for its treatment.
Melasma. Cay?rl? et al48 used the RegenKit® for three sessions at 15-day intervals and Garg et al49 used the two-spin centrifugation method for six sessions to administer PRP injections for the treatment of melasma. Both of these studies found significant melasma reductions upon PRP injection. TGF-?1 and PDGF present in PRP could have led to melasma reduction. TGF-?1 decreases melanogenesis via delayed extracellular signal-regulated kinase activation and therefore inhibits melanin synthesis in a concentration-dependent manner. PDGF could accelerate neogenesis, collagen synthesis, and ECM formation, leading to a favorable microenvironment in the dermis.
Psoriasis. Nuclear factor kappa B (NF-?B), which plays a regulatory role in inflammation, could also be a crucial mediator in the pathogenesis of psoriasis. PRP exerts inhibitory effect on NF-?B through enhanced cellular I?B? expression, which results in the retention the of NF-?B-p65 subunit within cytosol and prevents its nucleocytoplasmic shuttling. PRP reduces chemotaxis by inhibiting chemokine transactivation and CXCR4 receptor expression, possibly controlling local inflammation. Patients with chronic plaque psoriasis treated with PRP and methotrexate showed a significant improvement in erythema, induration, and desquamation at each visit compared to patients treated with methotrexate alone.50 At Week 16, all patients in the combination therapy group achieved a Psoriasis Area Severity Index (PASI) score of 50, 10 of 16 (62.5%) achieved PASI 75, and 2 of 16 (12.5%) achieved PASI 90. None of the patients in the monotherapy group achieved PASI 50, although they showed a 35 percent to 40 percent improvement relative to baseline PASI.50
Inflammatory nail disorder. Kaur et al51 reported intramatricial PRP injection to be a safe and effective alternative in refractory nail disorder. They reported one case with lichen striatus who was nonresponsive to six-week topical corticosteroid treatment and developed onychoschizia and leukonychia after two intramatricial steroid injections. Another case with idiopathic trachyonychia showed no improvement after eight-week topical corticosteroid treatment. Both cases showed significant improvement after two PRP injections administered at three-week intervals. The authors proposed that PRP improved inflammatory nail disorders by decreasing the inflammatory factors, thereby normalizing the nail matrix kinetics (both proliferation and keratinization) and promoting regeneration through the release of GFs.51
Conclusion
With the unveiling of its mechanisms and clinical efficacy in recent years, PRP seems to be a promising therapeutic modality in the field of dermatology. PRP, used alone or in combination with other therapies, showed beneficial effects in terms of cosmetic improvements and for some skin diseases. Only transient or short-term side effects were noted in some cases. Currently, there are no standardized PRP preparations, which are essential for optimizing this treatment. The role of leukocytes in PRP products for autoimmune diseases, including AA, vitiligo, and psoriasis, remains unclear and should be further explored and verified. Lastly, because PRP as a treatment option has become popular, more studies are required to elucidate its benefits and limitations.
References
- Alves R, Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord. 2018;4(1): 18–24.
- Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158–167.
- Hesseler MJ, Shyam N. Platelet-rich plasma and its utilities in alopecia: a systematic review. Dermatol Surg. 2019;81(3):834–846.
- DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28(7):998–1009.
- Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225–228.
- Dohan Ehrenfest DM, Andia I, Zumstein MA, et al. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4(1):3–9.
- Magalon J, Chateau AL, Bertrand B, et al. DEPA classification: a proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc Med. 2016;2(1):e000060.
- Kim DH, Je YJ, Kim CD, et al. Can platelet-rich plasma be used for skin rejuvenation? Evaluation of effects of plate-let-rich plasma on human dermal fibroblast. Ann Dermatol. 2011;23(4):424–431.
- Omar NN, Rashed RR, El-Hazek RM, et al. Platelet-rich plasma-induced feedback inhibition of activin A/follistatin signaling: a mechanism for tumor-low risk skin rejuvenation in irradiated rats. J Photochem Photobiol B. 2018;180:17–24.
- Abuaf OK, Yildiz H, Baloglu H, et al. Histologic evidence of new collagen formulation using platelet rich plasma in skin rejuvenation: a prospective controlled clinical study. Ann Dermatol. 2016;28(6):718–724.
- Everts PA, Pinto PC, Girão L. Autologous pure platelet-rich plasma injections for facial skin rejuvenation: Biometric instrumental evaluations and patient-reported outcomes to support antiaging effects. J Cosmet Dermatol. 2019;18(4):985–995.
- Cameli N, Mariano M, Cordone I, et al. Autologous pure platelet-rich plasma dermal injections for facial skin rejuvenation: clinical, instrumental, and flow cytometry assessment. Dermatol Surg. 2017;43(6):826–835.
- Alam M, Hughart R, Champlain A, et al. Effect of platelet-rich plasma injection for rejuvenation of photoaged facial skin: a randomized clinical trial. JAMA Dermatol. 2018;154(12):1447–1452.
- Elnehrawy NY, Ibrahim ZA, Eltoukhy AM, Nagy HM. Assessment of the efficacy and safety of single platelet-rich plasma injection on different types and grades of facial wrinkles. J Cosmet Dermatol. 2017;16(1):103–111.
- Lee ZH, Sinno S, Poudrier G, et al. Platelet rich plasma for photodamaged skin: a pilot study. J Cosmet Dermatol. 2019;18(1):77–83.
- Gawdat HI, Tawdy AM, Hegazy RA, et al. Autologous platelet-rich plasma versus readymade growth factors in skin rejuvenation: a split face study. J Cosmet Dermatol. 2017;16(2):258–264.
- Sclafani AP. Platelet-rich fibrin matrix for improvement of deep nasolabial folds. J Cosmet Dermatol. 2010;9(1):66–71.
- Wang X, Yang Y, Zhang Y, Miron RJ. Fluid platelet-rich fibrin stimulates greater dermal skin fibroblast cell migration, proliferation, and collagen synthesis when compared to platelet-rich plasma. J Cosmet Dermatol. 2019;18(6):2004–2010.
- Singh B, Goldberg LJ. Autologous platelet-rich plasma for the treatment of pattern hair loss. Am J Clin Dermatol. 2016;17(4):359–367.
- Gentile P, Garcovich S, Scioli MG, et al. Mechanical and controlled PRP injections in patients affected by androgenetic alopecia. J Vis Exp. 2018;(131):56406.
- Uebel CO, da Silva JB, Cantarelli D, Martins P. The role of platelet plasma growth factors in male pattern baldness surgery. Plast Reconstr Surg. 2006;118(6):1458–1466.
- Lee SH, Zheng Z, Kang JS, et al. Therapeutic efficacy of autologous platelet-rich plasma and polydeoxyribonucleotide on female pattern hair loss. Wound Repair Regen. 2015;23(1):30–36.
- Gentile P, Garcovich S, Bielli A, et al. The effect of platelet-rich plasma in hair regrowth: A randomized placebo-controlled trial. Stem Cells Transl Med. 2015;4(11):1317–1323.
- Alves R, Grimalt R. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2018;44(1):132–133.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42(11):1243–1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: A pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18(8):452–455.
- Tawfik AA, Osman MAR. The effect of autologous activated platelet-rich plasma injection on female pattern hair loss: a randomized placebo-controlled study. J Cosmet Dermatol. 2018;17(1):47–53.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28(1):72–79.
- Cole JP, Cole MA, Insalaco C, et al. Alopecia and platelet-derived therapies. Stem Cell Investig. 2017;4:88.
- Ince B, Yildirim MEC, Dadaci M, et al. Comparison of the efficacy of homologous and autologous platelet-rich plasma (PRP) for treating androgenic alopecia. Aesthetic Plast Surg. 2018;42(1):297–303.
- Stevens HP, Donners S, de Bruijn J. Introducing platelet-rich stroma: Platelet-rich plasma (prp) and stromal vascular fraction (svf) combined for the treatment of androgenetic alopecia. Aesthet Surg J. 2018;38(8):811–822.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42(11):1243–1247.
- Trink A, Sorbellini E, Bezzola P, et al. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br J Dermatol. 2013;169(3):690–694.
- Albalat W, Ebrahim HM. Evaluation of platelet-rich plasma vs intralesional steroid in treatment of alopecia areata. J Cosmet Dermatol. 2019 May 10. Epub ahead of print.
- Shumez H, Prasad PVS, Kaviarasan P, Deepika R. Intralesional platelet rich plasma vs intralesional triamcinolone in the treatment of alopecia areata: a comparative study. Int J Med Res Heal Sci. 2015;4(1):118–122.
- Singh S. Role of platelet-rich plasma in chronic alopecia areata: Our centre experience. Indian J Plast Surg. 2015;48(1):57–59.
- Donovan J. Successful treatment of corticosteroid-resistant ophiasis-type alopecia areata (AA) with platelet-rich plasma (PRP). JAAD Case Rep. 2015;1(5):305–307.
- Thamer Mubki. Platelet-rich plasma combined with intralesional triamcinolone acetonide for the treatment of alopecia areata: a case report. J Dermatol Dermatol Surg. 2016;20(1):87–90.
- El Taieb MA, Ibrahim H, Nada EA, Seif Al-Din M. Platelets rich plasma versus minoxidil 5% in treatment of alopecia areata: a trichoscopic evaluation. Dermatol Ther. 2017;30(1):e12437.
- Finney R. Commentary on use of platelet-rich plasma in cicatricial alopecia. Dermatol Surg. 2019;45(7):982–983.
- Parambath N, Sharma VK, Parihar AS, et al. Use of platelet-rich plasma to suspend noncultured epidermal cell suspension improves repigmentation after autologous transplantation in stable vitiligo: a double-blind randomized controlled trial. Int J Dermatol. 2019;58(4):472–476.
- Mahajan R, Ninama K, Shah H, Bilimoria F. Effect of intralesional platelet rich plasma in chronic localized vitiligo. Int J Res Dermatol. 2018;4(4):550–555.
- Ejjiyar M, Sahibi M, El Gueouatri M, et al. [Vitiligo and Koebner phenomenon following platelet-rich plasma injections]. Pan Afr Med J. 2019;32:58. Article in French.
- Garg S, Dosapaty N, Arora AK. Laser ablation of the recipient area with platelet-rich plasma-enriched epidermal suspension transplant in vitiligo surgery: a pilot study. Dermatol Surg. 2019;45(1):83–89.
- Abdelghani R, Ahmed NA, Darwish HM. Combined treatment with fractional carbon dioxide laser, autologous platelet-rich plasma, and narrow band ultraviolet B for vitiligo in different body sites: a prospective, randomized comparative trial. J Cosmet Dermatol. 2018;17(3):365–372.
- Kadry M, Tawfik A, Abdallah N, et al. Platelet-rich plasma versus combined fractional carbon dioxide laser with platelet-rich plasma in the treatment of vitiligo: a comparative study. Clin Cosmet Investig Dermatol. 2018;11:551–559.
- Ibrahim ZA, El-Ashmawy AA, El-Tatawy RA, Sallam FA. The effect of platelet-rich plasma on the outcome of short-term narrowband-ultraviolet B phototherapy in the treatment of vitiligo: a pilot study. J Cosmet Dermatol. 2016;15(2):108–116.
- Cay?rl? M, Cal??kan E, Aç?kgöz G, et al. Regression of melasma with platelet-rich plasma treatment. Ann Dermatol. 2014;26(3):401–402.
- Garg S, Khillan K, Bharija SC. Platelet-rich plasma therapy in the treatment of recalcitrant melasma. Dermatol Surg. 2019;45(3):482–484.
- Chakravdhanula U, Anbarasu K, Verma VK, Beevi SS. Clinical efficacy of platelet rich plasma in combination with methotrexate in chronic plaque psoriatic patients. Dermatol Ther. 2016;29(6): 446–450.
- Kaur I, Jakhar D. Intramatricial platelet-rich plasma therapy: A novel treatment modality in refractory nail disorders. Dermatol Ther. 2019;32(2):e12831.

