J Clin Aesthet Dermatol. 2021;14(11):E64–E75.
J Clin Aesthet Dermatol. 2021;14(11):E64–E75.
by Suparuj Lueangarun, MD, MSc; Poom Visutjindaporn, MD; Yardnapar Parcharoen, PhD; Pollawat Jamparuang, BSc; and Therdpong Tempark, MD
Drs. Lueangarun and Visutjindaporn are with the Division of Dermatology, Chulabhorn International College of Medicine, Thammasat University, in Pathum Thani, Thailand. Dr. Parcharoen is with the Chulabhorn International College of Medicine, Thammasat University in Pathum Thani, Thailand. Dr. Jamparuang is with the Radiometry Laboratory, Light and Color Group, Thermometry and Optical Metrology Department, National Institute of Metrology, Ministry of Higher Education, Science, Research and Innovation in Pathum Thani, Thailand. Dr. Tempark is with the Department of Pediatrics, King Chulalongkorn Memorial Hospital in Bangkok, Thailand.
FUNDING: No funding was provided for this article.
DISCLOSURES: The author reports no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Low-level light/laser therapy (LLLT) can potentially stimulate hair growth in pattern hair loss (PHL), with many available home-use LLLT devices of different designs and technology on the market. However, not all devices are cleared by the United States (US) Food and Drug Administration (FDA), with very few studies to support their efficacy.
Objectives. This systematic review and meta-analysis aimed to investigate the effectiveness of FDA-approved LLLT devices for PHL treatment.
Methods. We included articles related to FDA-approved home-use LLLT devices on PubMed and Medline, using the FDA 510(K) Premarket Notification database and the systematic search of articles up to January 2020. The standardized mean difference (SMD) for the changes of hair density treated by LLLT versus sham devices was analyzed.
Results. Only 32 home-use LLLT devices have been approved by the FDA as of January 2020. The meta-analysis comprised seven double-blinded, randomized, controlled trials. The overall quantitative analysis yielded a significant increase in hair density in those treated by LLLT versus sham groups (SMD: 1.27, 95% confidence interval [CI]: 0.993–1.639). The subgroup analysis demonstrated the increased hair growth in male and female subjects with both comb- and helmet-type devices. There were significant LLLT sources in the LDs alone (SMD: 1.52, 95% CI: 1.16–1.88) and the LDs combination (SMD: 0.85, 95% CI: 0.55–1.16) (p=0.043).
Conclusion. LLLT is potentially effective for PHL treatment. Nonetheless, the long-term follow-up study in patients with severe PHL with combined standard treatment and comparison between LLLT devices and energy sources is recommended.
Key words: Pattern hair loss, low-level laser therapy, low-level light therapy, FDA-approved LLLT devices, androgenetic alopecia, hair loss
Pattern hair loss (PHL), also called androgenetic alopecia (AGA), affects half of the men aged 50 years or older and more than half of the women aged 80 years or older.1 Although PHL is not fatal, many patients lose their confidence and become stressed.2
Oral finasteride and topical minoxidil2 in various forms, including solution, foam, and shampoo, are currently approved by the United States (US) Food and Drug Administration (FDA) for PHL treatments. Despite its treatment efficacy, oral finasteride can cause some unfavorable side effects, such as erectile dysfunction, decreased libido, and increased body hair growth.3,4 Thus, other treatment options are recommended in those who respond poorly or experience undesirable side effects.5
In 1967, a pioneer research study was first conducted to assess the “photobiostimulation” or low-level light/laser therapy (LLLT) on mice by using the ruby laser.6 Results indicated increased hair growth on the shaved-off areas of animals’ backs. The efficacy of LLLT has since been investigated by many scientists, and the utilization of LLLT therapy has been adapted worldwide, especially for certain skin diseases, such as AGA.
The mechanism of LLLT in hair regrowth enhances the stimulation of mitochondria located in hair bulge stem cells, with cytochrome c oxidase (CCO) in the membrane of the mitochondria as the target chromophore of red light. This then leads to mitochondrial respiration, with reactive oxygen species (ROS) and adenosine triphosphate (ATP) to activate cellular proliferation, migration, and oxygenation, which consequently promote hair growth.7
In 2007, the first cleared LLLT device was introduced for male patients with PHL by the FDA. Since then, the numerous forms of convenient LLLT technology and devices have been modified by different manufacturers in the current US market, though some of those LLLT products with very few published articles to support their efficacy have been approved by the FDA. Thus, we aimed to investigate the efficacy of the FDA-approved LLLT devices, with different designs and light/laser sources, on hair growth in male and female patients. Also, the information on decisions of both physicians and patients regarding the most appropriate LLLT devices and specific technology was reviewed.
Methods
Literature search. A search using product code “OAP” from the FDA 510(k) Premarket Notification Medical Database for all home-use LLLT devices was performed.8 The devices were sconsidered safe and effective, substantially equal to legally marketed devices. OAP was categorized as “laser, comb, hair” product code to promote hair growth. The identification of all devices in the US market was reviewed and combined with information on the manufacturers’ websites.
A search of PubMed and Medline for articles related to the devices was performed through January 5, 2020. The search terms included “low-level light therapy” OR “low-level laser therapy” OR “photobiomodulation (PBM)” OR “HairMax LaserComb” AND “androgenetic alopecia” OR “female PHL” OR “male PHL” OR “PHL” OR “hair growth.”
Inclusion and exclusion criteria. Only the studies on AGA or PHL in humans and the use of FDA-approved devices, written in English, were included. The review articles, studies on animals, usage of other modalities, not commercially available in the market, and not written in English were excluded.
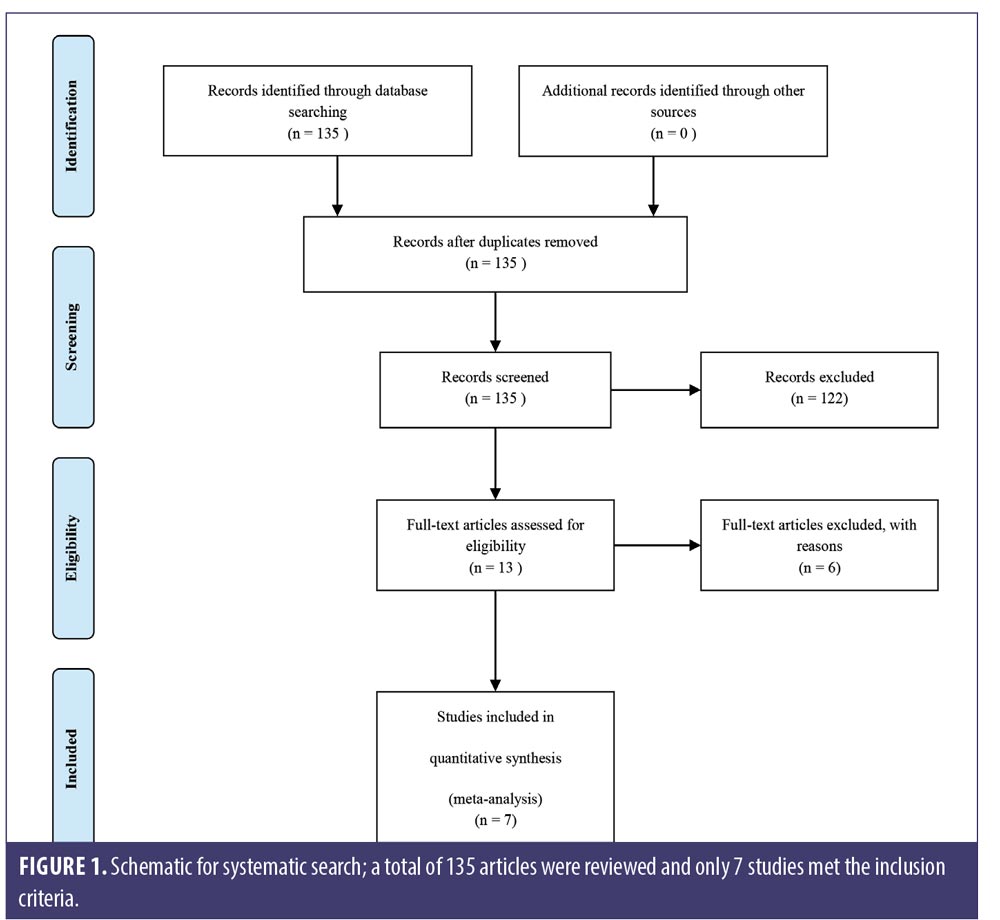
All search results were screened by two reviewers for relevant abstracts and titles. Full texts of potentially relevant studies were thoroughly examined for the eligibility of final inclusion. The results were independently reviewed by two authors. Any discrepancies were discussed among all authors for inclusion and exclusion criteria. We excluded three studies of nonavailable devices,9–11 one study comparing LLLT with topical minoxidil with no sham control,12 one retrospective observational study,13 and one prospective nonrandomized control trial.8 Seven studies met the inclusion criteria for our meta-analysis (Figure 1).
Study quality assessment. The methodological quality of included studies was independently evaluated by two reviewers, using Jadad scoring for the evaluation of randomized control trials (RCTs)14 in three aspects: randomization (2 points), blinding (2 points), and accountability of all patients (1 point). Potential scores ranged from 0 to 5, with a higher score indicating better methodological quality. Discrepancies between reviewers were resolved through discussions.
Data extraction. Data on study design, type of intervention, and outcome were extracted by two reviewers and screened for eligible studies in duplicate according to titles, abstracts, and full texts.
Information on interventions and device properties was gathered by first author, year, sample size, number and type of treatment arms, participant characteristics, comparative arm regimens, and summary of general LLLT protocol. Data on design of device, type and number of light/laser sources, wavelength, irradiation parameter, irradiation time, session frequency (days/weeks) and treatment duration (weeks), retail cost, and special features were also collected.
The type of outcome measurement was described (unit area trichogram, phototrichogram, global photography, direct hair count, hair analysis software, and blinded or nonblinded investigator for hair analysis), as well as primary endpoints (hair count/density, hair thickness/shaft diameter, vellus hair count/density, terminal hair count/density, anagen percentage, telogen percentage, tensile strength, and investigator global assessment), and secondary endpoints (patient satisfaction and subject global assessment).
Data synthesis and analysis. The standardized mean differences (SMDs) of hair density between the LLLT and the sham groups were the primary outcome.15,16 The positive SMD value of LLLT indicated a more favorable treatment option when compared to sham. The model of random effects was employed to pool individual SMDs. All analyses were performed using Stata/MP 16 software (StataCorp LP, College Station, TX, USA). Between-trial heterogeneity was determined by using I2 tests. The two-tailed p-value, I2 values >50 percent, and p-value <0.05, were considered as significant heterogeneity.14,17 Funnel plots and Egger’s test were used to examine potential publication bias.14,16 Statistical significance was defined as p-values <0.05.
Results
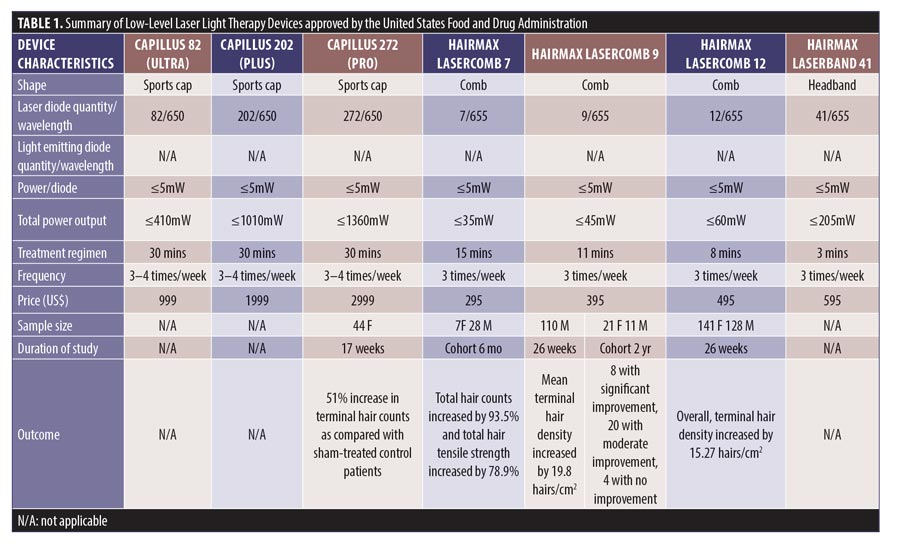
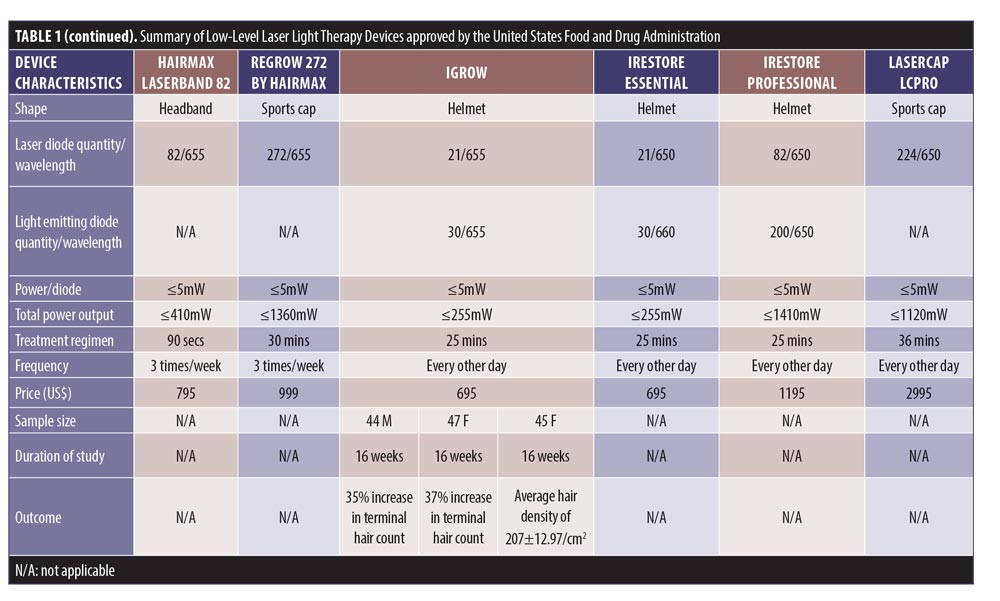
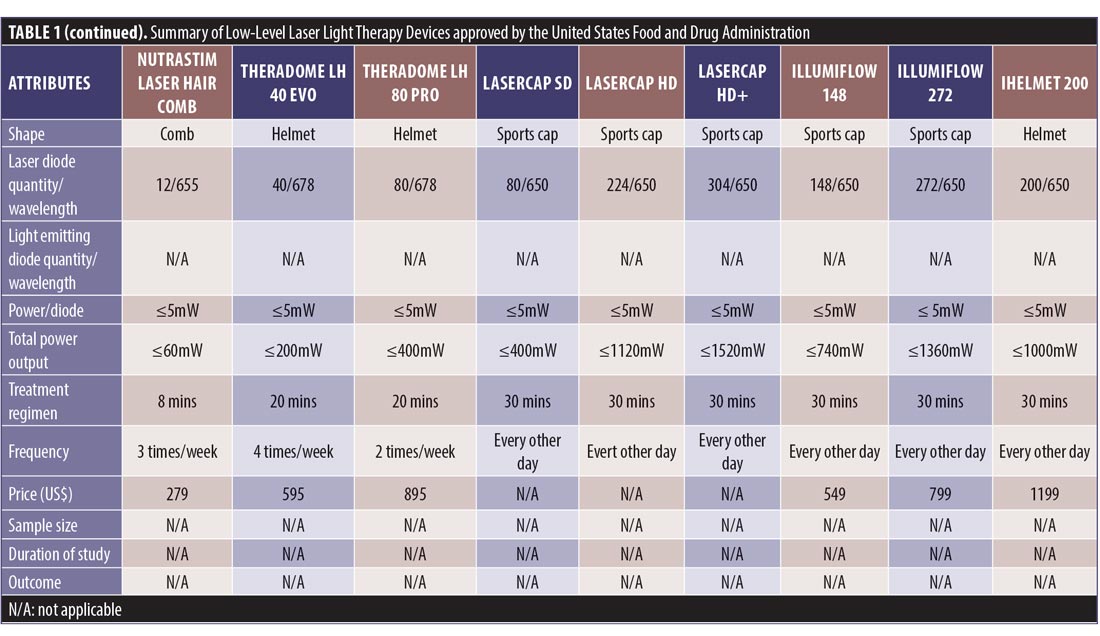
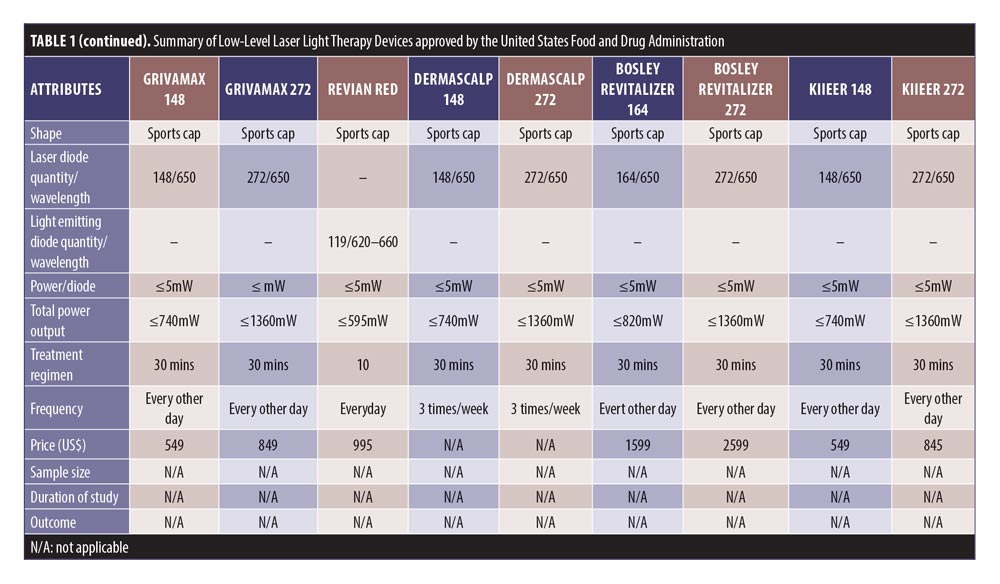
Study search and characteristics of included patients. There were 58 entries in the FDA 510(k) Premarket Notification Medical Database. Of these, 32 home-use LLLT devices were marketed in the US up to January 5, 2020 (Table 1). Nevertheless, only some of them had the published documents to support their efficacy. A few had the supporting clinical trials but no publications in the journals.
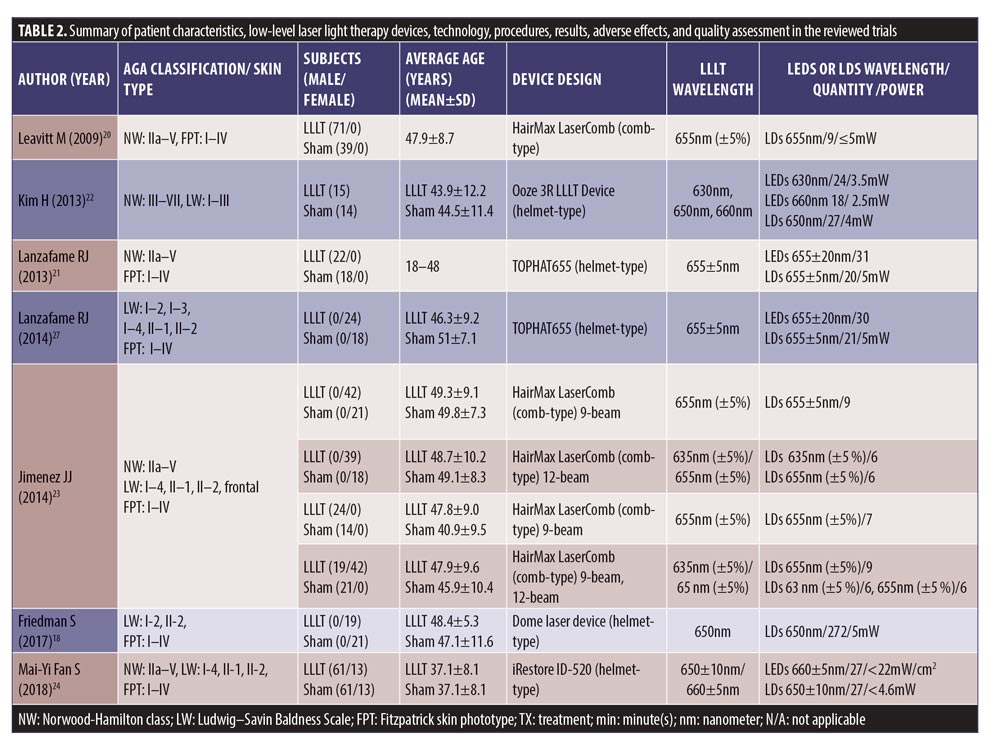
We retrieved 135 nonduplicated articles after the meticulous evaluation of titles and abstracts. Those not meeting the inclusion criteria were eliminated (Figure 1). Thus, a total of seven studies (RCTs) were included in the meta-analysis. The efficacy of LLLT devices was compared between patient populations of the RCTs and the controls. Friedman et al (2017)18 and Lanzafame et al (2014)19 conducted their studies only on women. Leavitt et al (2009)20 and Lanzafame et al (2013)21 included only men in their studies. The three RCTs by Kim et al (2013),22 Jimenez et al (2014),23 and Mai-Yi Fan et al (2018)24 included both sexes. The study by Jimenez et al (2014)23 had the largest number of participants (128 male and 141 female). The rest of the articles had small sample sizes. The quantitative analysis included 607 participants. Patient characteristics, results, and quality assessment of those trials are summarized in Table 2.
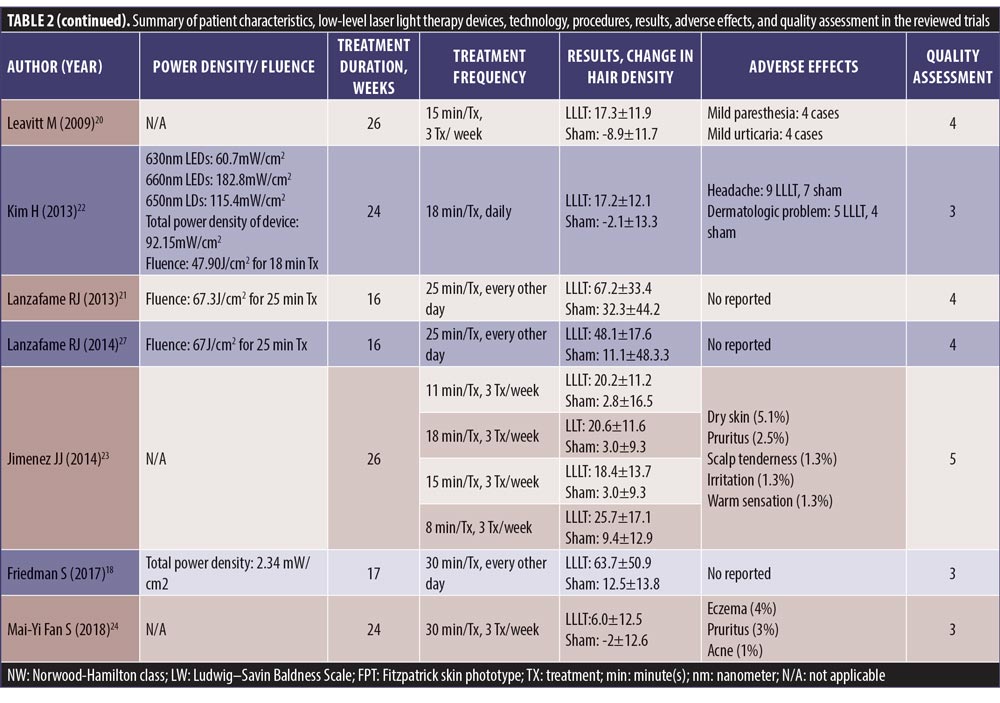
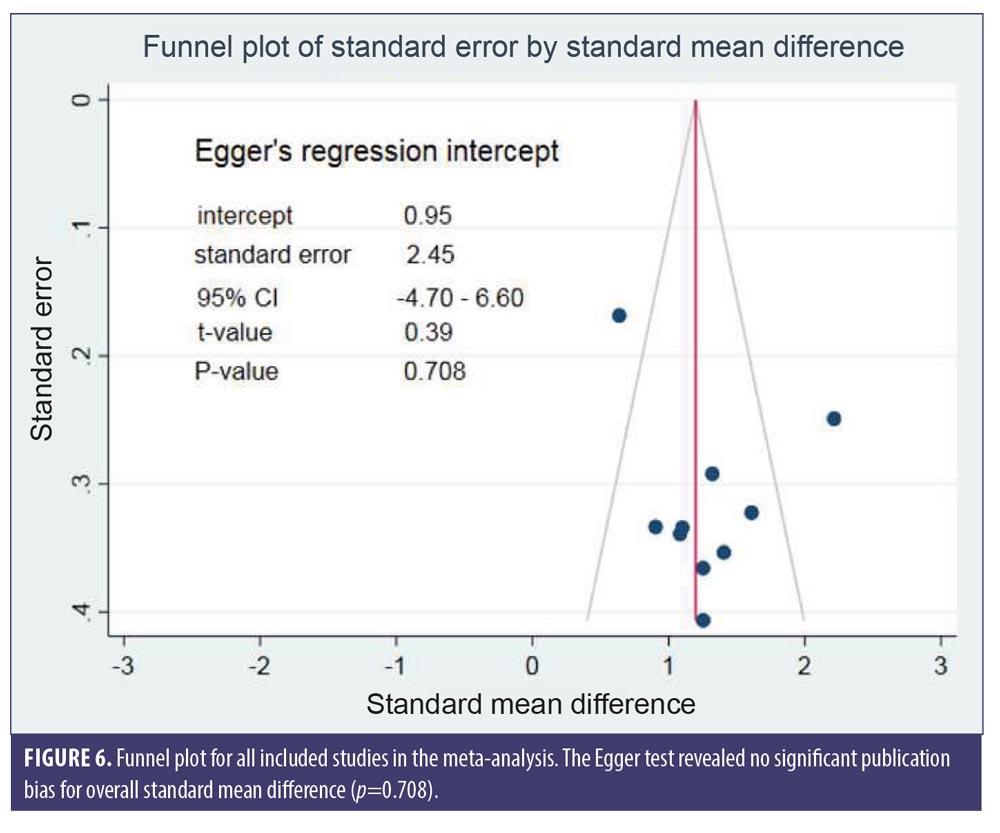
SMDs of changes in hair density. All studies evaluated the changes in hair density. There was a statistically significant increase of hair density in the LLLT group. The overall SMD of LLLT versus sham on the changes in hair density was 1.27 (95% confidence interval (CI): 0.96–1.59), as shown in Figure 2. The SMD heterogeneity of I2 was 64.17 percent.
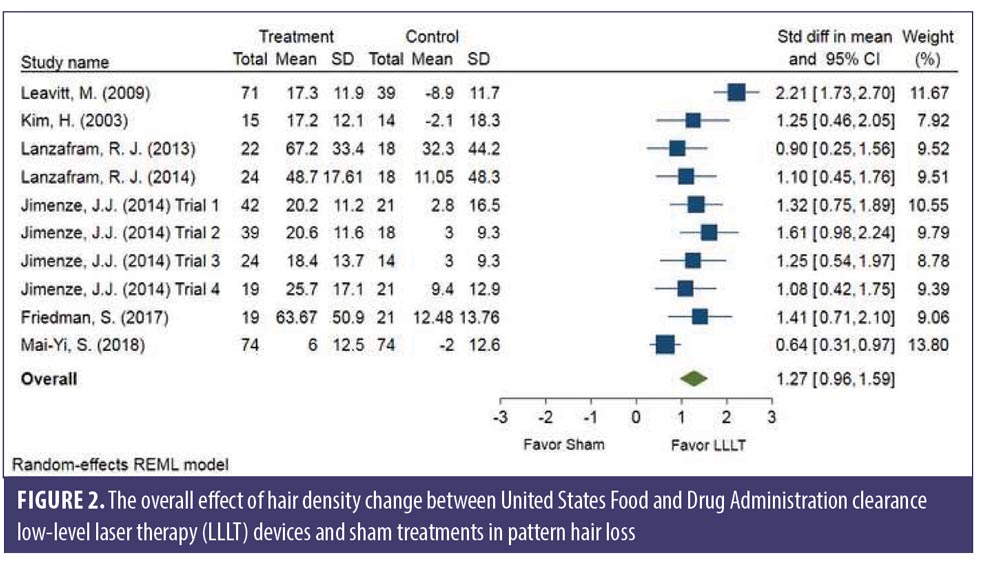
Subgroup analysis comparing each sex. No significant difference was observed between sexes. The changes in hair density of LLLT versus sham in the male (SMD: 1.40, 95% CI: 0.78–2.01) and the female (SMD: 1.36, 95% CI: 1.04–1.68) groups is shown in Figure 3. The SMD heterogeneity of I2 was less than 0.01 percent in the female group and 74.30 percent in the male group (Figure 3).
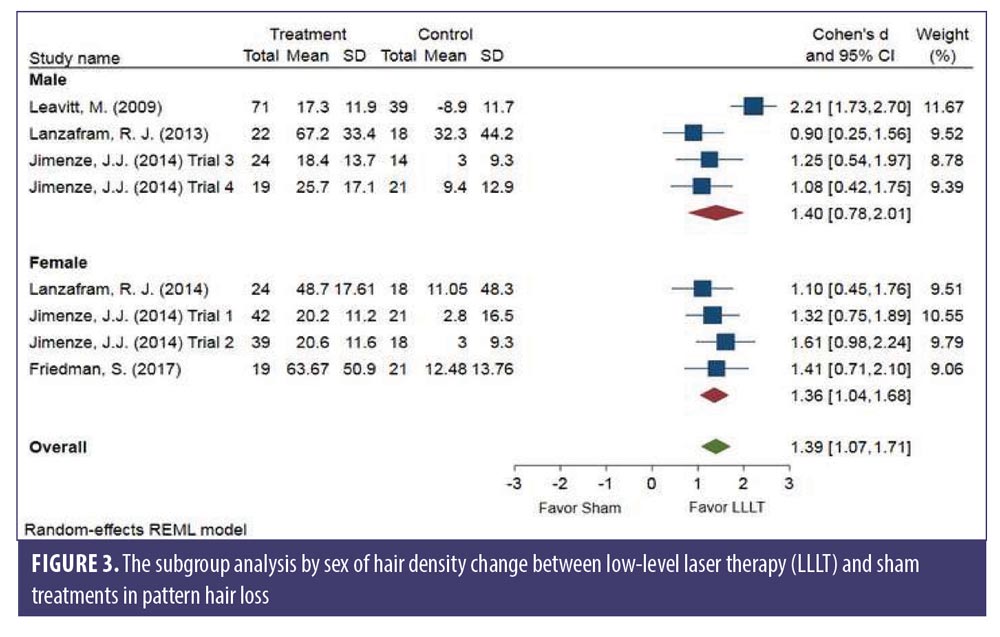
Subgroup analysis comparing LLLT devices and LLLT procedures. There was no significant difference between the comb- and helmet-type LLLT devices. The changes in hair density of LLLT versus sham in the comb-type (SMD: 1.53, 95% CI: 1.11–1.96) and the helmet-type (SMD: 0.97, 95% CI: 0.64–1.29) groups is shown in Figure 4. The SMD heterogeneity of I2 was less than 58.99 percent in the comb-type group and 35.09 percent in the helmet-type group (Figure 4).
Subgroup analysis comparing LLLT light/laser sources. A significant difference was demonstrated between LLLT light and laser sources (p=0.043). The changes in hair density of LLLT versus sham in the LDs alone group (SMD: 1.52, 95% CI: 1.16–1.88) and in LEDs and LDs combination groups (SMD: 0.85, 95% CI: 0.55–1.16) is shown in Figure 5. The SMD heterogeneity of I2 was 51.25 percent in the LDs group, and 20.15 percent in the LEDs and LDs combination groups (Figure 5).
Egger’s test illustrated no significant publication bias for the overall SMD (p=0.708). The funnel plot for SMD of changes in hair density is shown in Figure 6.
LLLT technological characteristics. Overall, there were four major designs of the devices, including sport cap (20 devices), headband (2 devices), comb (4 devices), and helmet (6 devices) according to the manufacturers. Each device had a number of LLLT sources. Of these, Revian Red® (REVIAN, Inc., Durham, NC, USA) was the only device with just LED of 620 to 640nm wavelength, while iGrow® (Apira Science, Inc., Boca Raton, FL, USA) and iRestore® (Freedom Laser Therapy Inc., Irvine, CA, USA) devices contained both LDs and LEDs. Twenty-eight of the devices contained only LDs, with the number ranging from seven LDs (HairMax Lasercomb® 7, Lexington International LLC., Boca Raton, FL, USA) to 304 LDs (LaserCap HD+, LaserCap Company, Highland Heights, OH, USA). The median number of LDs used in these devices was 148 (range 7–304), with power less than 5mW. Nevertheless, the median (range) total output of these devices was 740mW (35–1520mW), with a wavelength of 650nm (620–678nm).
The treatment regimens averaged 30 minutes (ranging from 90 seconds to 36 minutes), depending on shapes and total power outputs. HairMax® LaserBand 82 (Lexington International LLC., Boca Raton, FL, USA) required the least treatment time of 90 seconds. Meanwhile, LaserCap® LCPRO (LaserCap Company, Highland Heights, OH, USA) needed the maximum treatment duration of 36 minutes. Most devices were advised to be used about 3 to 4 times per week. Only Revian Red® was instructed to be used daily.
The retail costs started from $279 to $2,999,25,26 which varied according to the numbers of LDs and special features, such as a mobile app connection,20 of each device (Figure 7).

Review of FDA-approved LLLT device characteristics and studies.
Capillus®. The Capillus272 Pro consists of 272 red, visible light of 650nm LDs. Each LD emits a power of 5mW, with a density of 2.34mW/cm2. The device is configured within an outer helmet and protective inner liner for portable use, a rechargeable battery, and an adapter, and it can automatically pause therapy. If the subject’s head moves outside of the radiation zone, the therapy will pause and resume when the correct head position reestablishes in a safety interlock, with audible tones indicating whether the therapy has begun or ended.
Only the published article by Friedman et al supported the Capillus® device (Curallux, LLC., Doral, FL, USA).18 In 2017, this multicenter, double-blind, RCT was conducted on 44 women (19 LLLT and 21 sham controls), using the sport cap LLLT or sham devices at home for 30 minutes per day on an alternate day for 17 weeks (total of 60 treatments). The total irradiance per treatment area per session was at 4.21J/cm2. The primary outcome showed a significant increase in terminal hair counts compared to baseline at the end of Week 17 for 63.67 percent versus 12.48 percent (p<0.001) in the LLLT and the sham groups, respectively. No adverse events were noted.
HairMax®. The HairMax LaserComb Advanced 7®, HairMax Lux 9®, and HairMax Professional 12® consist of hand-held LLLT devices. Each device provides the distributed laser light to the scalp. The comb teeth can bypass the laser to reach the scalp. The device emits a beep (9 and 12 laser models) or vibration (7 laser models) every four seconds to indicate the user should move the device to a new section of the scalp.
There were four published articles supporting the HairMax® devices. In 2003, Satino et al8 first conducted a prospective study in 35 patients (7 female and 28 male) using the 655nm HairMax Lasercomb® at home. All patients were instructed to comb their hair approximately one-fourth of an inch per second for 5 to 10 minutes on an alternate day for six months. The study showed a 93.5 percent increase in terminal hair counts compared to baseline and a 78.9 percent increase in hair tensile strength.
In 2009, Leavitt et al20 conducted a double-blinded, sham-controlled study in the recruited 110 men with PHL (71 HairMax Lasercomb® and 39 sham-controlled devices). The device composed of 9 LDs, with emitting power at 5mW and wavelength of 655+5nm. Patients were instructed to use this sham-controlled device three times per week for 15 minutes over 26 weeks. The primary outcome yielded a significant increase of 19.8 hair/cm2 for mean terminal hair counts in the HairMax Lasercomb® group and a decrease of 7.6 hair/cm2 in the sham-controlled group (p<0.0001).
Munck et al (2014)13 conducted a retrospective observational study to evaluate the efficacy and safety of LLLT for PHL, either monotherapy or concomitant therapy with minoxidil or finasteride in an office-based setting. A total of 32 patients (11 men and 21 women) were instructed to use HairMax Lasercomb® three times a week at home. The duration was 8 to 15 minutes per session, depending on the purchased model, with different numbers of LDs (7, 9, or 12). Each LD had a 655nm wavelength with power density of 5mW. The mean duration of treatment was 8.7 months±5.2. Of 32 patients, eight (25%) showed significant improvement, 20 (62.5%) moderate improvement, and four (12.5%) no improvement from the global photographic assessment. Improvement was observed in both monotherapy and concomitant therapy, as early as three months and up to a maximum time of 24 months.
In 2014, Jimenez et al23 performed a randomized, multicenter, double-blind, sham-controlled study in 128 men and 141 women with PHL, using the HairMax Lasercomb®. The patients were randomized to receive the comb device with seven, nine, or 12 LDs, or the sham device, three times a week for 8 to 15 minutes per session, depending on the model, for 26 weeks (8 minutes for 12-laser diode model, 11 minutes for 9-laser diode model, and 15 minutes for 7-laser diode model). Each LD emitted a wavelength of 655nm, with a power density of 5mW. The primary endpoint was a significant increase of terminal hair density for 15.27 hair/cm2 compared to the sham-controlled group at a 26-week follow-up session (p<0.001).
iGrow®. The iGrow-II Hair Growth System consists of 21 red visible light LDs and 30 red LEDs configured within an outer helmet and protective inner liner. The use of LDs and nonlaser LEDs provides a full coverage of the upper one-third of the head (i.e., the area commonly covered with stylized hair). The helmet system automatically pauses the therapy if the subject’s head moves outside of the zone of radiation. The therapy will resume when the correct head position is re-established. At the end of each cycle, the system signals the therapy is complete and is ready to be powered down by emitting an audible beep pattern.
In 2013, Lanzafame et al21 conducted an RCT of LLLT for treatment of PHL in 41 men, using the helmet device containing 21 LDs and 30 LEDs with wavelength of 655+5nm every other day for 16 weeks. The patients were instructed to use this device for 25 minutes per session, with a power density of 2.9J/cm2. The control group used an identical unit containing incandescent lights. The primary endpoint demonstrated a significant increase in hair counts for 35 percent over the control group (p=0.003).
In 2014, Lanzafame et al19 also conducted an RCT on 42 women, using the same device as in 2013. The primary outcome was the percentage of increase in hair counts between two groups, which yielded an increase of 37 percent (p<0.0001). No side effects were reported.
In 2017, the study of LLLT in combination with standard treatment was evaluated by Esmat et al12 to compare the effects of LLLT, minoxidil, and combination therapy in 45 women. All subjects were categorized into three groups, including a 5% minoxidil group, LLLT group using iGrow®, and combination therapy group. In the minoxidil group, each patient was advised to use topical minoxidil two times a day for four months. In the LLLT group, the subjects were treated with LLLT device for 25 minutes daily for four months. In the combination therapy group, patients were instructed to use both topical minoxidil and LLLT. The minoxidil, the LLLT alone, and the combination-treated groups showed a significant increase of hair density for 34.94 percent, 34.41 percent, and 43.69 percent of participants at four months, respectively (p<0.001). Only the combination-treated group demonstrated a statistically significant improvement by physician assessment (p<0.001). No major side effects were detected in all patients throughout the full duration of treatment.
iRestore®. The iRestore Essential consists of 21 red visible light LDs and 30 red LEDs, while the iRestore Professional 282 comprises of 82 LDs and 200 LEDs. Both were configured within an outer helmet and protective inner liner. The use of LDs and non-laser LEDs provides a full coverage of the upper one-third of the head (i.e., the area commonly covered with stylized hair). If the subject’s head moves outside of the radiation zone, the helmet system will automatically pause the therapy and resume when the correct head position is re-established. At the end of the cycle, the system signals the therapy is complete by emitting an audible beep pattern.
The study by Kim et al22 was the first to evaluate different effects of this LLLT device for both male and female patients with PHL. Forty subjects (14 female and 26 male) were enrolled in the 24-week randomized, double-blind, sham device-controlled multicenter study. Patients received either a helmet type home-use LLLT or a sham device for use 18 minutes daily for 24 weeks. This helmet-type LLLT device is composed of a light source with LEDs emitting a wavelength of 630nm (3.5mW, 24 units) and 660nm (2.5mW, 18 units), and LDs emitting a wavelength of 650nm (4mW, 27 units). All of the diodes run simultaneously through six cycles, each consisting of two minutes and 50 seconds on and 10 seconds off. There was an increase of hair counts measured by the phototrichogram for 14.68 percent versus -1.68 percent (p<0.05) at Week 24 in the LLLT and the control groups, respectively.
In 2018, Mai-Yi Fan et al24 conducted a 24-week, randomized, double-blind, self-comparison, sham device-controlled trial, which enrolled 100 patients with PHL. All patients were randomly assigned to receive the investigational LLLT on one side of the head and the sham light treatment on the other side three times weekly for 30 minutes each over a 24-week period. The iRestore ID-520 (WELLMIKE Technology Corp., New Taipei City, Taiwan) was a helmet-type LLLT device with a light source of LEDs array of 660nm (<22mW/cm2, 27 LED) and a LDs array of 650nm (<4.6 mW, 27 pieces). After 24 weeks of treatment, the LLLT-treated scalp exhibited a significantly greater hair coverage than the sham light-treated side (14.2% vs. 11.8%, p<.001).
LLLT and other treatment combinations. In 2014, a retrospective observational study by Munck et al13 was conducted in both male and female participants with AGA to evaluate the efficacy and safety of comb LLLT devices, either monotherapy or concomitant therapy, with minoxidil or finasteride, in an office-based setting. The global improvement was observed in both monotherapy and concomitant therapy. Of 26 patients in the concomitant therapy group, six (23.1% ) showed significant improvement, 16 (61.5%) moderate improvement, and four (15.4%) no improvement. However, there was no statistically significant difference between the LLLT monotherapy and the concomitant therapy with either minoxidil and/or finasteride (p=0.829) in male or female participants with AGA (p=0.091).
Another RCT compared different effects in the minoxidil, LLLT, and combination-treated groups of female patients with PHL for four months,12 showing a significant increase of hair density in all groups (p<0.001). Moreover, the physician assessment indicated 90 percent of the combined treatment group showed improvement, with 100 percent reported patient satisfaction.
Discussion
Despite many available LLLT devices of various designs and technology for PHL treatment in the US market, only a few of them have been approved by the FDA, with only some studies of specific devices published in PubMed database to support their efficacy.
This present meta-analysis focused on the treatment efficacy of the FDA cleared home-use LLLT devices on hair density improvement in PHL patients. We included seven RCTs18,20–24,27 in PHL patients who were treated by the FDA-approved LLLT devices. There was a significant increase in hair density in PHL patients with LLLT treatment when compared to the sham controls. Interestingly, the LLLT devices could improve hair density in both men and women, with no significant difference between the two sexes. Additionally, both the helmet-type and the comb-type LLLT devices yielded effective treatment outcomes, yet with a significant difference between light and laser sources.
In the systematic review, there were 32 home-use LLLT devices approved by the FDA in the current market. Thus, our systemic review and meta-analysis proposed to compare the efficacy of those devices with controls in seven RCTs, including one Capillus®,18 two HairMax®,20,23 two iGrow®,21,27 and two iRestore®.22,24 Moreover, there were two cohort studies, one prospective cohort study8 and one retrospective cohort study,13 published in PubMed database to support the efficacy of home-use LLLT devices.
The subjects in most RCTs had mild-to-moderate PHL (Norwood-Hamilton Class IIa–V for men and Ludwig-Savin Baldness Scale I–2, I–3, I–4, II–1, II–2 for women). The duration of the studies ranged from 16 to 26 weeks, with a mean duration of 21.3 weeks.
Most articles in RCTs showed positive results of an increase in microscopic hair counts. The majority of LLLT treatment groups yielded a statistically significant improvement in hair counts compared to the control group. In two noncontrolled trials,8,13 there was an improvement in the LLLT group compared to baseline. One RCT studied the efficacy of a helmet LLLT device in combination with topical minoxidil in female participants with PHL.12
The differences in designs of those LLLT devices included shape, light source, number of LEDs and LDs, wavelength, and total power output. Due to the lack of a head-to-head study, we were unable to evaluate the clinical benefits of one device over another. When considering the number of diodes, the comb-type LLLT devices used the least number of diodes, ranging from 7 to 12 diodes. The prices of comb-type LLLT devices were lower than other shapes and also demonstrated clinical efficacy.21,24 Hence, they could be good options for patients with financial limitations. The hand-free devices including caps18 and helmets,12,21,27 which demonstrated clinical efficacy, usually used a high number of diodes ranging from 40 to 304 with user-friendly capability. In particular, patients could manage their daily activities during treatment sessions.
Among 32 home-use LLLT devices, the majority (87.5%) were composed of only LDs, with essential lasers for therapeutic benefits. Following the collimated, coherent, highly monochromatic beam properties, the possibility of high power densities were then considered preferable.28 Interestingly, our meta-analysis revealed a significant difference between light and laser sources. However, the noncoherent light sources, such as LEDs and broad-band lamps, have become more prevalent, with advantages of LEDs, including no laser safety issues, ease of home use, ability to irradiate a large area of tissue at once, wearability, and much lower cost per mW.
A range of light energy sources from LEDs to lasers have been used with specific advantages and limitations.29 There is evidence to show the nondependence of photobiomodulation on lasers or coherence, while the quasimonochromatic LED devices could also yield physiological effects.28
The study of LLLT devices consisted of 224 red LDs (660nm, 5mW) in the treatment group, while the sham devices composed of 224 LEDs (650+20nm) in the control group. Each LD had the optical power at the irradiance of 3.5mW/cm2, whereas the LEDs comprised the power of 0.5mW/diode (10 times lower than LDs). There was an increase in hair density and diameter in both groups. The treatment group was significantly superior to the sham devices, with the mean change from baseline to Week 24 for hair density of 10.21±3.25 hairs/cm2 in the treatment group versus 3.95±1.32 hairs/cm2 in the sham group. The mean change for hair diameter was 6.11±2.15?m in the laser group versus 3.76±1.24?m in the sham group. Therefore, the LEDs were as likely to impact photobiomodulation as the LDs.
Due to the very low power of LEDs (0.5mW/diode), compared to the appropriate dose of 2 to 4J/cm2, the results of LEDs could not be obviously concluded.30 Nevertheless, some head-to-head studies, which compared both light sources, yielded no difference in their effects.28 Hence, more high-quality head-to-head comparison studies are required to verify the significant difference between dose responses or physiological effects of LEDs and laser PBM.12,29
All devices used the light/laser sources with 620 to 678nm following the absorption peak at 660nm31 of CCO as the chromophore for LLLT. According to Arndt-Schulz Law, it is widely accepted that there is no response in the case of the irradiance or the too-short duration.32 Likewise, with the irradiance or too-high duration, the response may be inhibited. According to Hamblin et al (2009)32 the irradiance of 2 to 4J/cm2 was determined to be appropriate. When this theory was applied to the LLLT devices, it usually provided patients with this therapeutic irradiance.
In a study to compare the efficacy of standard treatments including oral finasteride and topical minoxidil with LLLT, the 1mg of finasteride in male patients with PHL for 12 months significantly increased total hair counts for 7.3 percent and 8.99 percent of the patients at six and 12 months, respectively (p<0.001).33 The 2% and 5% topical minoxidil demonstrated a significant increase of nonvellus hair counts for 8.84 percent (p=0.013) and 12.3 percent (p<0.001) at 48 weeks, respectively.34 The LLLT efficacy revealed a significant increase of terminal hair counts for 20.9/cm2 (12.79%, p=0.0249) versus 25.7/cm2 (16.96%, p=0.0028) in the 9- and 12-beam laser comb treated side at 26 weeks after treatment, respectively.23 Hence, the efficacy of LLLT appears comparable to the conventional PHL treatment. The comparable efficacy was also observed in treatment of female patients with PHL for four months. The combination treatment of 5% minoxidil and LLLT appeared to provide a better response of hair density than minoxidil or LLLT alone.
From the published articles to support the efficacy of devices, the included participants had only mild-to-moderate PHL. However, no study was conducted in severe cases of PHL, an area that requires further investigation. In terms of duration of studies, no RCT was conducted longer than 26 weeks. Thus, the current recommendation for or against the treatment of more than six months with LLLT in PHL could not be affirmed at present.2 Nonetheless, the long-term efficacy in the real setting has not yet been investigated. Initially, the duration of treatment depends on the power density of the device. The treatment duration should be short with a high-power density device. The irradiance (power multiplied by duration time) should be within the therapeutic range (2–4J/cm2).32 Unfortunately, only the RCTs by Friedman et al (2017),18 Lanzafame et al (2013),21 and Lanzafame et al (2014)19 reported the irradiance use in their studies.
Limitations. Only a few RCTs of LLLTs in PHL treatment were conducted in a short-term follow-up (maximum 26 weeks). Also, there were limited amounts of trials with large sample sizes, while the wavelength and power used in LLLTs were diverse within the enrolled trials. Particularly, no head-to-head studies with efficacy comparison between the devices were observed.
Conclusion
Following our systematic review, the design of devices and the technology of home-use LLLT devices approved by the FDA in the current US market could be divided into four main categories: cap, comb, hairband, and helmet, with LDs being more popular than LEDs for the wavelength within the range of 620 to 678nm. From the meta-analysis, LLLT could improve hair density for both male and female patients with PHL, with no significant difference between sexes and comb- and helmet-type devices for the increase of hair density, indicating promising efficacy of LLLT devices. Interestingly, there was a significant difference of improvement of hair density between energy sources. Due to the short duration of studies (<26 weeks), overall, the devices should be further evaluated in the longer-term studies, especially in patients with severe PHL. Moreover, head-to-head studies among the devices and different LLLT sources, in combination with standard treatments, such as oral finasteride, should be conducted to further elucidate their efficacy.
Acknowledgments
The authors would like to extend special thanks to Ms. Sunattee Kessung for her assistance in editing and revising this manuscript and Ms. Kamonwan Soonklang for her statistical analysis.
References
- Gan DC, Sinclair RD. Prevalence of male and female pattern hair loss in Maryborough. Paper presented at: Journal of Investigative Dermatology Symposium Proceedings, 2005.
- Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men–short version. J Eur Acad Dermatol Venereol. 2018;32(1):11–22.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39(4):578–589.
- Panchaprateep R, Lueangarun S. Efficacy and safety of oral minoxidil 5mg once daily in the treatment of male patients with androgenetic alopecia: an open-label and global photographic assessment. Dermatol Ther (Heidelb). 2020;10(6):1345–1357.
- Lueangarun S, Panchaprateep R. An herbal extract combination (biochanin A, acetyl tetrapeptide-3, and ginseng extracts) versus 3% minoxidil solution for the treatment of androgenetic alopecia: a 24-week, prospective, randomized, triple-blind, controlled trial. J Clin Aesthet Dermatol. 2020;13(10):6.
- Mester E, Szende B, Tota J. Effect of laser on hair growth of mice. Kiserl Orvostud. 1967;19:628–631.
- Hamblin MR. Photobiomodulation for the management of alopecia: mechanisms of action, patient selection and perspectives. Clin Cosmet Investig Dermatol. 2019;12:669–678.
- Satino JL, Markou M. Hair regrowth and increased hair tensile strength using the HairMax LaserComb for low-level laser therapy. Int J Cosmet Surg Aesthetic Dermatol. 2003;5(2):113–117.
- Barikbin B, Khodamrdi Z, Kholoosi L, et al. Comparison of the effects of 665nm low level diode haser hat versus and a combination of 665nm and 808nm low level diode laser scanner of hair growth in androgenic alopecia. J Cosmet Laser Ther. 2017;10.1080/14764172.2017.1326609.
- Blum K, Han D, Madigan MA, et al. “Cold” X5 Hairlaser used to treat male androgenic alopecia and hair growth: an uncontrolled pilot study. BMC Res Notes. 2014;7:103.
- Avram MR, Rogers NE. The use of low-level light for hair growth: part I. J Cosmet Laser Ther. 2009;11(2):110–117.
- Esmat SM, Hegazy RA, Gawdat HI, et al. Low level light-minoxidil 5% combination versus either therapeutic modality alone in management of female patterned hair loss: a randomized controlled study. Lasers Surg Med. 2017;49(9):835–843.
- Munck A, Gavazzoni MF, Trüeb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6(2):45.
- Mella JM, Perret MC, Manzotti M, et al. Efficacy and safety of finasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146(10):1141–1150.
- Delaney SW, Zhang P. Systematic review of low-level laser therapy for adult androgenic alopecia. J Cosmet Laser Ther. 2018;20(4):229–236.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(1):136–141.e5.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32(12):2112–2125.
- Friedman S, Schnoor P. Novel approach to treating androgenetic alopecia in females with photobiomodulation (low-level laser therapy). Dermatol Surg. 2017;43(6):856–867.
- Lanzafame RJ, Blanche RR, Chiacchierini RP, et al. The growth of human scalp hair in females using visible red light laser and LED sources. Lasers Surg Med. 2014;46(8):601–607.
- Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb® laser phototherapy device in the treatment of male androgenetic alopecia. Clin Drug Investig. 2009;29(5):283–292.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45(8):487–495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39(8):1177–1183.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15(2):115–127.
- Mai-Yi Fan S, Cheng YP, Lee MY, et al. Efficacy and safety of a low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, self-comparison, sham device-controlled trial. Dermatol Surg. 2018;44(11):1411–1420.
- NutraStim. Available at: http://nutrastimhair.com. Accessed Jan 3, 2020.
- Capillus. Available at: https://www.capillus.com. Accessed Dec 28, 2019.
- Lanzafame RJ, Blanche RR, Chiacchierini RP, et al. The growth of human scalp hair in females using visible red light laser and LED sources. Lasers Surg Med. 2014;46(8):601–607.
- Heiskanen V, Hamblin MR. Photobiomodulation: lasers vs. light emitting diodes? Photochem Photobiol Sci. 2018;17(8):1003–1017.
- Mosca RC, Ong AA, Albasha O, et al. Photobiomodulation therapy for wound care: a potent, noninvasive, photoceutical approach. Adv Skin Wound Care. 2019;32(4):157–167.
- Suchonwanit P, Chalermroj N, Khunkhet S. Low-level laser therapy for the treatment of androgenetic alopecia in Thai men and women: a 24-week, randomized, double-blind, sham device-controlled trial. Lasers Med Sci. 2019;34(6):1107–1114.
- Karu T, Kalendo G, Letokhov V, et al. Biostimulation of HeLa cells by low-intensity visible light. Il Nuovo Cimento D. 1982;1(6):828–840.
- Huang Y-Y, Chen AC-H, Carroll JD, et al. Biphasic dose response in low level light therapy. Dose Response. 2009;7(4):358–383.
- Roberts JL, Fiedler V, Imperato-McGinley J, et al. Clinical dose ranging studies with finasteride, a type 2 5?-reductase inhibitor, in men with male pattern hair loss. J Am Acad Dermatol. 1999;41(4):555–563.
- Olsen EA, Dunlap FE, Funicella T, et al. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002;47(3):377–385.