J Clin Aesthet Dermatol. 2021;14(6):31–34.
J Clin Aesthet Dermatol. 2021;14(6):31–34.
by Aikaterini Gkouvi, MD; Electra Nicolaidou, MD, PhD; Andreas Corbo, MD; Gennaro Selvaggi, MD; Antonis Tsimpidakis, MD; Styliani Mastraftsi, MD; and Stamatios Gregoriou, MD, PhD
Dr. Gkouvi is in private practice in Thessaloniki, Greece. Drs. Nicolaidou, Tsimpidakis, Mastraftsi, and Gregoriou are with the First Department of Dermatology-Venereology, Faculty of Medicine at the National and Kapodistrian University of Athens in Athens, Greece. Dr. Corbo is in private practice in Rome, Italy and with the University of Tor Vergata in Rome, Italy. Dr. Selvaggi is with the Department of Plastic Surgery at the Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg and Sahlgrenska University Hospital in Gothenburg, Sweden.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. There is still an unsatisfied need for new treatments for vitiligo with more rapid onset and long-term sustainability of repigmentation.
Objective. We sought to evaluate the possible efficacy of heterologous type I collagen as an add-on therapy to narrowband ultraviolet B (NB-UVB) for the treatment of vitiligo.
Methods. Five patients with non-segmental vitiligo older than 18 years with bilateral and approximately symmetrical vitiligo lesions that did not evolve in size for at least six months were included. All vitiligo lesions were treated with NB-UVB therapy according to the Vitiligo Working Group recommendations. Two selected nonfacial lesions of each patient were also treated with intradermal injections of heterologous type I collagen (HTIC) every two weeks. Repigmentation of HTIC plus NB-UVB-treated lesions and their symmetrical counterparts treated just with NB-UVB was evaluated at baseline and Week 12.
Results. Repigmentation of the HTIC-injected lesions started after the first treatment session in three cases and after the second session in two cases. After six sessions (Week 12), the mean repigmentation rate was 70.5 percent (95% confidence interval:0.569–0.841) in the NB-UVB plus HTIC treatment group versus 16.5 percent (95% confidence interval: 0.137–0.192) in NB-UVB treatment group (p=0.0006, paired t-test).
Conclusion. Although the number of patients treated with the combination treatment was limited in our study, our results suggest that the addition of HTIC to NB-UVB therapy might offer a more rapid onset of repigmentation in patients with vitiligo.
Keywords: Vitiligo, heterologous type I collagen, narrowband ultraviolet B, phototherapy, collagen, ultraviolet
Vitiligo is a relatively common, acquired disease characterized by a progressive loss of functional melanocytes, which results in the appearance of well-circumscribed white macules and patches on the skin. Several mechanisms to date have been investigated to explain melanocyte destruction, including genetic and autoimmune factors, oxidative stress, inflammatory mediators, and melanocyte detachment mechanisms.1 However, these theories are insufficient to provide an undisputable mechanism of pathogenesis for all vitiligo phenotypes. Consequently, an “integrated theory” suggests that multiple mechanisms might work jointly in vitiligo to contribute to the destruction of melanocytes, ultimately leading to the same clinical result.
Although a variety of treatments for the repigmentation of vitiligo lesions is available, none effectively promote complete and long-lasting repigmentation. Narrowband ultraviolet B phototherapy (NB-UVB) has been established as a safe and efficacious treatment for generalized nonsegmental vitiligo.2 However, the response to NB-UVB varies among patients and repigmentation might only appear after several months of treatment. Thus, the combination of NB-UVB and another treatment modality could be used in an effort to achieve faster and greater repigmentation.
The promotion of melanocyte proliferation and melanogenesis enhancement has been suggested as a prospective vitiligo therapeutic strategy and therapeutic modalities such as platelet-rich plasma intradermal injections have been used in the treatment of vitiligo, with promising results.3 Furthermore, the disappearance of melanocytes has been suggested to be the final step of a complex scenario of deregulated biological events involving keratinocytes and fibroblasts as well.4 Heterologous type I collagen (HTIC) has been shown to promote cell and fibroblast proliferation and improve cutaneous extracellular matrix metabolism.5,6 Thus, intradermal injections of HTIC may influence the repigmentation process in vitiligo lesions.
The objective of this pilot case series was to evaluate the potential synergistic effect of HTIC intradermal injections in combination with NB-UVB in stable, nonsegmental vitiligo.
Methods
Five female patients with stable nonsegmental vitiligo were included in this study. Study inclusion criteria were age older than 18 years, bilateral and symmetrical vitiligo lesions, presence of lesions that did not evolve in size for at least six months, no new lesions appearing during the study period, lack of koebnerization, and no history of keloids or hypertrophic scarring.
All patients had been previously treated with topical treatment, including topical potent steroids and calcineurin inhibitors, with minimal or no response. A three-month washout period from any therapy was undertaken before study enrollment. All patients showed a willingness and ability to comply with the study requirements, and signed a written informed consent form.
All vitiligo lesions were treated with NB-UVB therapy according to the Vitiligo Working Group recommendations.2 The initial dose was at 200mJ/cm2, with a subsequent increase of 10 to 20 percent per session. Sessions were held three times per week for 12 weeks (n=36 exposures total). The dose was individualized for each patient following the development of mild erythema.
Two selected nonfacial lesions of each patient were additionally treated with intradermal injections of HTIC (Linerase®; Euroresearch, Milano, Italy). Linerase® is the commercial brand name of 100-gr micronized sterile powder of HTIC in vials for injectable use, obtained by type I collagen extracted from equine tendons. Tripeptides obtained from the polymer hydrolysis are composed of proline, hydroxyproline, and glycine.
Each of the treated lesions had an approximately symmetrical counterpart lesion that was treated with only NB-UVB. Patients completed a total of six HTIC injection sessions over a period of three months. Patients were followed up with for 12 months after the last NB-UVB session. For each HTIC session, 100mg of HTIC (micronized, sterile powder dissolved in 4.5mL of normal saline [0.9% sodium chloride solution]) and 0.5mL of lidocaine were injected into the randomly chosen affected areas. The infiltration pattern that was followed was to inject 0.1mL of this solution intradermal (or directly subdermal) in a grid pattern at approximately 2-cm intervals over the entire treated area. Injections were carried out using 30-gauze needles (length: 4mm). No local anesthesia was used.
Patients were evaluated after each injectable treatment regarding the progress of therapy, the presence of any side effects, and pain during the treatment session. At each follow-up assessment, the extent of repigmentation within each treated patch was estimated to the nearest of one of the following percentages: 0, 10, 25, 50, 75, 90, or 100 percent. A paired t-test was performed for the comparison of mean improvement in repigmentation between the group treated with NB-UVB plus HTIC and the group treated just with NB-UVB. The STATA/SE version 11 software program (StataCorp LLC, College Station, Texas) was used for statistical analysis and calculations
Results
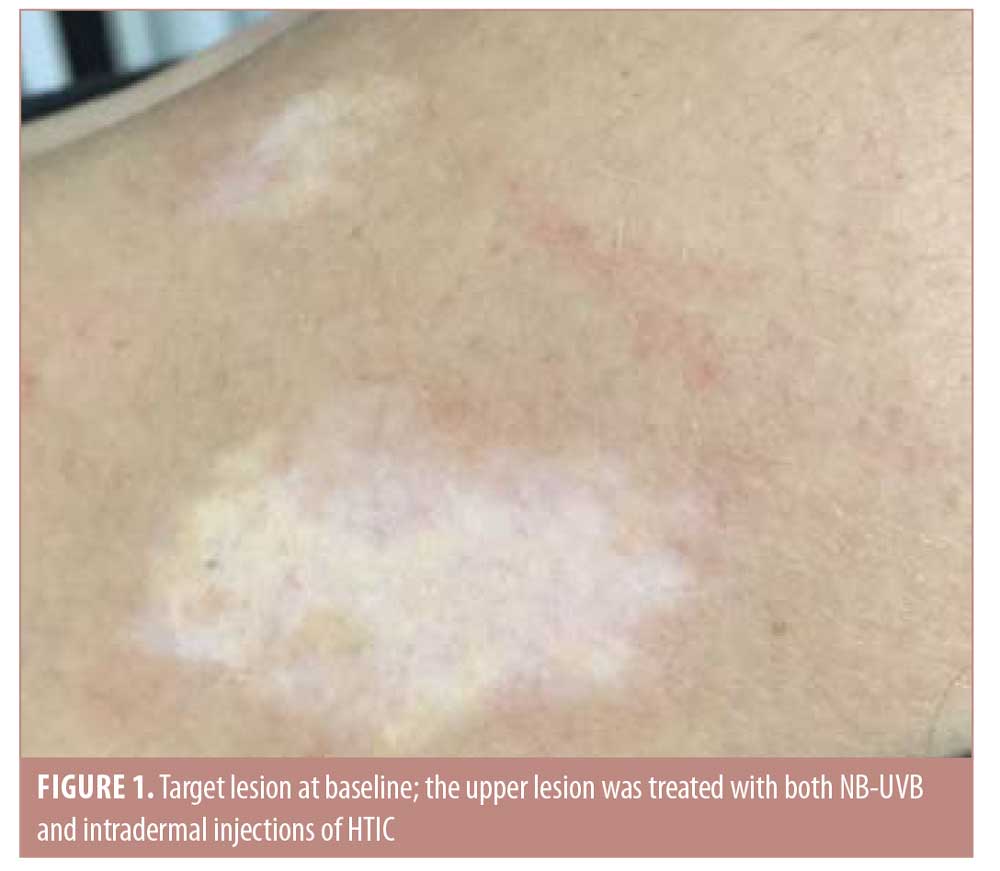
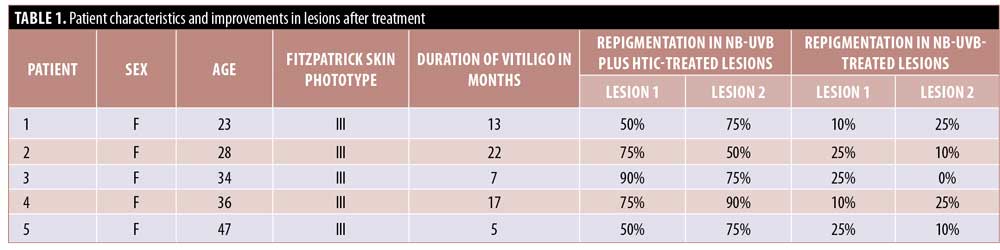
Characteristics of the five patients included in the study are presented in Table 1. Repigmentation at the HTIC-injected lesions started after the first session in three cases and after the second session in two cases. This presented as perifollicular pigmentation at injection-treated areas that subsequently progressed to fully repigmented patches.
After six sessions of HTIC injections (12th week of the treatment period), a statistically significant improvement was observed in favor of NB-UVB plus HTIC group (Figures 1 and 2). The mean improvement was 70.5 percent (95% confidence interval: 0.569–0.841) in the NB-UVB plus HTIC treatment group versus 16.5% (95% confidence interval: 0.137–0.192) in the NB-UVB group (p = 0.0006, paired t-test). Repigmentation results for all lesions are provided in Table 1.
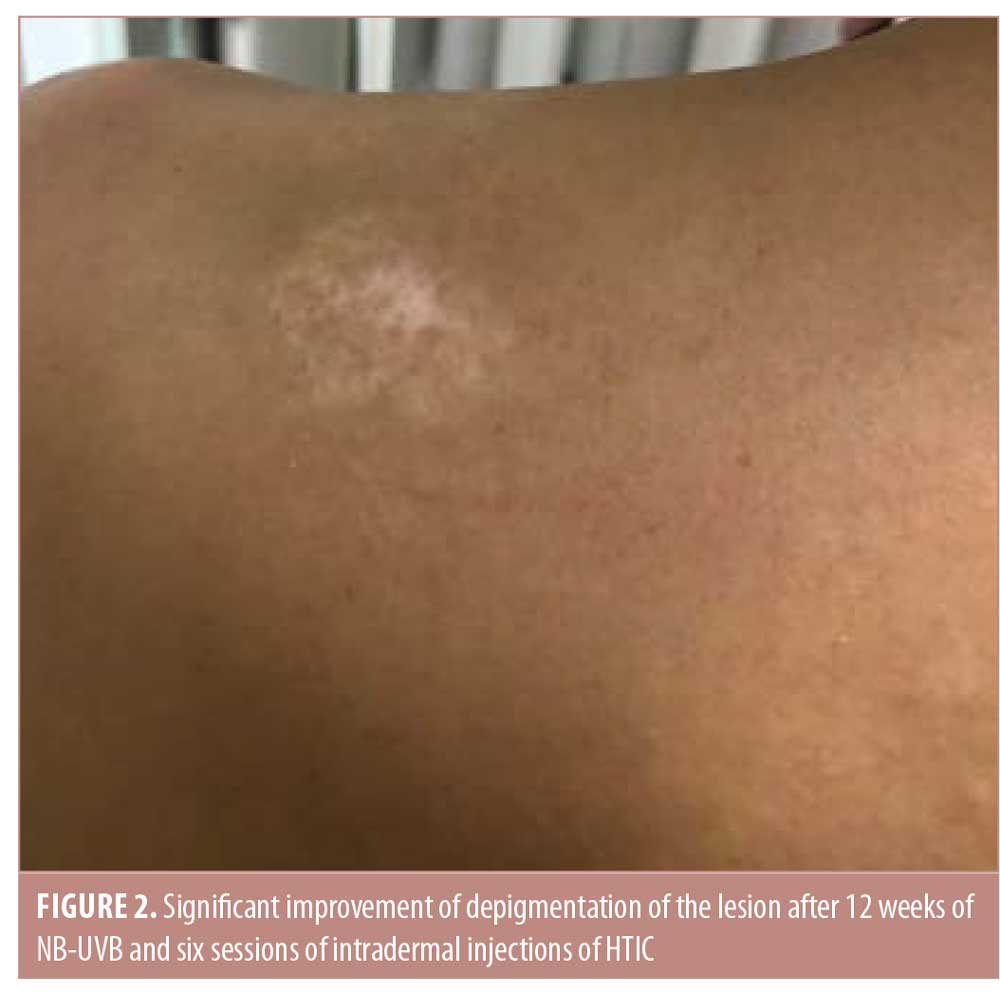

All patients reported minimal to moderate pain during the collagen injection procedure. There were no adverse events. No relapses were reported during follow up to 12 months after the final NB-UVB treatment.
Discussion
The initial results in this exploratory use of HTIC as an add-on therapy to NB-UVB for the treatment of vitiligo are encouraging, suggesting that this approach might trigger a more rapid response in patients undergoing phototherapy.
The possible mechanism of action of HTIC in vitiligo repigmentation is difficult to illuminate. The dermis is a source of signaling proteins acting on epithelial cells via extracellular matrix components and fibroblasts. Fibroblasts influence epidermal pigmentation, either through a direct activity on melanin distribution/degradation or activation of keratinocytes for the production of melanogenic factors.7 Mediators synthesized by fibroblasts, such as hepatocyte growth factor (HGF), keratinocyte growth factor (KGF), cytokine stem cell factor (CSF), basic fibroblast growth factor (bFGF, FGF2), neurotrophin-3 (NT-3), semaphorin 7a (Sema7a), transforming growth factor-beta (TGF-beta), and fibroblast activation protein-alpha (FAP-alpha), bind to their specific receptors and thereby modulate melanocyte-related intracellular signaling pathways (MAPK/ERK, cAMP/PKA, Wnt/beta-catenin, PI3K/Akt) related to melanocyte functions. These factors influence the growth and the pigmentation of melanocytes via the expression of melanin-producing enzymes and melanosome transfer, as well as their dendricity, mobility, and adhesive properties.8
Collagen-derived peptides have been shown to stimulate cell proliferation, stimulate stem cells, and downregulate the release of inflammatory mediators.6 In an in-vitro study, Ohara et al5 showed that proline-hydroxyproline derived from collagen significantly increases the activity of dermal fibroblasts.5 Jennings et al9 reported that collagen fragments, significantly inhibit the attachment of chondrocytes to the matrix and matrix degradation induction. Schneider et al10 cultivated melanocytes from the outer root sheath of human hair follicles on artificial extracellular matrix coatings containing collagen and hyaloronan with varying sulfation degrees. The matrix enabled elevated gene expression of melanotic markers, including melanotic genes MITF, C-KIT, TYR, and PMEL68–70 and melanin production relative to the control polysterene surface.10 In addition, it has been suggested that collagen fragments might exercise an immunomodulatory role by inducing regulatory T-cells that affect macrophage polarization toward M2-like macrophages and the suppression of an immune response.11,12 It is possible that these properties of HTIC might limit melanocyte apoptosis and enhance proliferation.
Limitations. The main limitations of this case series include the limited number of patients and a lack of data on the use of HTIC as monotherapy. Larger, randomized controlled trials are needed to determine the possible role of HTIC in the treatment of vitiligo. In addition, future studies should also address the issue of whether the observed improvement could be partially due to the tissue needling involved in the collagen injection process, since micro-needling has been suggested to induce skin cell proliferation and release propigmentary cytokines in vitiligo patients.13
Conclusion
Although the number of patients treated with the combination treatment was limited in our study, our results suggest that the addition of HTIC to NB-UVB therapy might offer a more rapid onset of repigmentation in patients with vitiligo.
References
- Abdel-Malek ZA, Jordan C, Ho T, et al. The enigma and challenges of vitiligo pathophysiology and treatment. Pigment Cell Melanoma Res. 2020;33(6):778–787.
- Mohammad TF, Al-Jamal M, Hamzavi IH, et al. The Vitiligo Working Group recommendations for narrowband ultraviolet B light phototherapy treatment of vitiligo. J Am Acad Dermatol. 2017;76(5):879–888.
- Khattab FM, Abdelbary E, Fawzi M. Evaluation of combined excimer laser and platelet-rich plasma for the treatment of non-segmental vitiligo: a prospective comparative study. J Cosmet Dermatol. 2020;19(4):869–877.
- Bastonini E, Bellei B, Filoni A, et al. Involvement of non-melanocytic skin cells in vitiligo. Exp Dermatol. 2019;28(6):667–673.
- Ohara H, Ichikawa S, Matsumoto H, et al. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J Dermatol. 2010;37(4):330–338.
- Avantaggiato A, Girardi A, Palmieri et al. Comparison of bio-revitalizing injective products: a study on skin fibroblast cultures. Rejuvenation Res. 2015;18(3):270–276.
- Cario-Andre M, Pain C, Gauthier Y, et al. In vivo and in vitro evidence of dermal fibroblasts influence on human epidermal pigmentation. Pigment Cell Res. 2006;19(5):434–442.
- Wang Y, Viennet C, Robin S, et al. Precise role of dermal fibroblasts on melanocyte pigmentation. J Dermatol Sci. 2017;88(2):159–166.
- Jennings L, Wu L, King KB, et al. The effects of collagen fragments on the extracellular matrix metabolism of bovine and human chondrocytes. Connect Tissue Res. 2001;42(1):71–86.
- Schneider M, Rother S, Möller S, et al. Sulfated hyaluronan-containing artificial extracellular matrices promote proliferation of keratinocytes and melanotic phenotype of melanocytes from the outer root sheath of hair follicles. J Biomed Mater Res A. 2019;107(8):1640–1653.
- Chen L, Bao B, Wang N, et al. Oral administration of shark type II collagen suppresses complete Freund’s adjuvant-induced rheumatoid arthritis in rats. Pharmaceuticals (Basel). 2012;5(4):339–352.
- Xi C, Tan L, Sun Y, et al. A novel recombinant peptide containing only two T-cell tolerance epitopes of chicken type II collagen that suppresses collagen-induced arthritis. Mol Immunol. 2009;46(4):729–737.
- Feily A, Firoozifard A, Sokhandani T, et al. Follicular transplantation, microneedling, and adjuvant narrow-band ultraviolet-B irradiation as cost-effective regimens for palmar-plantar vitiligo: a pilot study. Cureus. 2020;12(4):e7878.