 J Clin Aesthet Dermatol. 2022;15(1):E61–E65.
J Clin Aesthet Dermatol. 2022;15(1):E61–E65.
by Michael H. Gold, MD; April Wilson, RN, BSN; and Julie A. Biron, BS
Dr. Gold is with Gold Skin Care Center in Nashville, Tennessee. Ms. Wilson and Ms. Biron are with Tennessee Clinical Research Center in Nashville, Tennessee.
FUNDING: Funding for this study was provided by GPQ srl.
DISCLOSURES: Dr. Gold is a consultant to GPQ Srl and Tennessee Clinical Research Center was compensated for performing this research.
ABSTRACT: Background. Chemical peeling with trichloroacetic acid (TCA) is a well-established modality to improve the appearance of chrono- and photodamaged skin.
Objective. We sought to evaluate the efficacy and safety of a novel formulation of TCA and hydrogen peroxide for the treatment of chrono- and photodamaged skin. Methods. Healthy subjects (N=5, 100% female) aged 49 to 72 years and Fitzpatrick Skin Types II (n=2) and III (n=3) enrolled in the Institutional Review Board-approved study. Subjects had mild to moderate facial wrinkles. Subjects were treated with a TCA-peroxide product four times at one-week intervals and applied at-home care products daily between visits. Investigator Global Assessments (IGA), investigator tolerability assessments, and subjective tolerability assessments were conducted on all subjects at each visit. The median score at each visit was calculated and compared to baseline using the Friedman test for repeated measures.
Results. Among IGA attributes, differences in median scores from baseline were significant among the four visits for tone (p=0.0174), smoothness (p=0.0014), texture (p=0.0017), redness, (p=0.0013) and overall appearance (p=0.0073), while the difference approached significance for radiance (p=0.0749). Twenty percent of subjects showed immediate improvement in radiance, tone, smoothness, and texture (Visit 1, immediately after the initial treatment) while 100 percent of subjects showed improvement by Visit 3 for smoothness, texture, and redness, and the improvements persisted to the end of the study. The treatment was well-tolerated and adverse events were not observed.
Conclusion. The TCA-peroxide and at-home care products appear to provide rapid and consistent improvement of chrono- and photodamaged skin without adverse effects.
Keywords: Hydrogen peroxide, wrinkles, rhytids, trichloracetic acid, skin stress
Chemical peeling was ranked among the top five minimally invasive cosmetic procedures in 2019 by The American Society of Plastic Surgeons. Also known as chemical exfoliation, chemical peeling causes controlled epidermal damage with or without part of the dermis, resulting in skin regeneration and remodeling. Chemical peels can be used to treat acne vulgaris, chrono- and photodamage, pigmentary disorders, and scars.1
Chemical peels may be superficial, medium, or deep, depending on their depth of destruction. Trichloroacetic acid (TCA) at concentrations less than 50% is a medium-depth peel that exfoliates the epidermis and upper layers of the dermis, including the papillary dermis.2
A novel formulation of TCA and hydrogen peroxide (33%) has been developed to improve the appearance of chrono- and photodamaged skin without precipitation of skin proteins. The TCA-peroxide product was designed to activate the “skin stress response system” (SSRS), a localized neuroendocrine pathway similar to the hypothalamic-pituitary-adrenal (HPA) axis for systemic stress.3,4 In the novel formulation, the action of TCA on the epidermis is partially neutralized by hydrogen peroxide while the remaining hydrogen ions move to the dermis where they promote cell proliferation and neocollagenesis.5
The present study evaluates the efficacy and safety of the TCA-peroxide formulation for the treatment of chrono- and photodamaged skin.
Methods
Study design. This was a single-center, open-label pilot study to evaluate the efficacy and safety of a novel TCA peel (PRX-T33 Perfect Intense Liquid TCA Peel; GPQ Srl, Milano, Italy) for the treatment of facial chrono- and photodamage. A secondary objective was to determine the improvement and conditioning of facial skin treated with posttreatment products (WiQo Dry Skin Cream and WiQo Smoothing Fluid; GPQ Srl).
Patients. Healthy subjects (N=5, 100% female) aged 49 to 72 years with Fitzpatrick Skin Types II (n=2) and III (n=3) enrolled in the International Review Board-approved study. Subjects had mild to moderate facial wrinkles of Glogau Grade II or III. Exclusion criteria were recent excessive exposure to sunlight or artificial ultraviolet (UV) light; recent or current facial skin condition which, in the opinion of the investigator, would interfere with the diagnosis or evaluation of the study parameters; use of isotretinoin within the past six months or retinoic acid within the past 15 days; treatments (surgical or non-surgical) in the target area within the past three months; melanoma or squamous cell carcinoma in the target area within the past five years; excessive hair or body adornments in the target area; allergy to product ingredients; participation in other clinical trials; or any mental or physical condition which, in the opinion of the investigator, compromised the safety of the participant in this study. Female participants of child-bearing age were required to produce a negative urine pregnancy test for inclusion in the study. All subjects provided signed informed consent to participate in this study.
Procedure. Subjects were screened for eligibility and, in some cases, treated on Day 0 (baseline). On the day of treatment, each patient was asked to remove all makeup and wash their face. The skin was cleansed with a cleanser (WiQo P solution; GPQ Srl) until a cotton swab showed no residue. TCA treatment product (2mL) was withdrawn with an 18-gauge needle or cannula and applied first to the forehead and then gradually downward to the jawline with constant massaging into the skin, taking care to keep the treated areas lubricated. Full-face application was repeated with up to three layers of the TCA treatment product until the skin was visibly firm and palpable. Only a single layer was applied to the eyelids, nasolabial fold, glabella, and marionette lines. Excess liquid was removed with cotton and subjects immediately washed their faces with cold running water. When the face was dry and clean, moisturizer (WiQo moisturizing Face Cream; GPQ Srl) was applied liberally to the treated areas. Subjects were given postcare products and a diary before leaving the office. Vials of the TCA-peroxide product, including those partially used, were stored at 2°C to 8°C.
Subjects were questioned about medications and adverse events at each of the five or six visits (screening, Days 0, 7, 14, 21, 51) and photographs were taken on Day 0, Day 21, and Day 51. Treatments and assessments (Investigator Global and subjective) were performed at each visit and satisfaction was assessed at the end of the study.
Home care. After full-face treatment, home care products and diaries were dispensed. Subjects were instructed to apply a skin cream (WiQo Dry Skin Cream; GPQ Srl) twice daily and a serum (WiQo Facial Smoothing Fluid; GPQ Srl) each evening, the latter at least 30 minutes prior to applying the skin cream. Subjects were asked to record each application in their diaries.
The skin cream that each participant was instructed to use is formulated with a high percentage of shea butter (14%) tht contains a comparatively high fraction (7%) of unsaponifiable lipids (i.e., triterpenes, tocopherol, phenols, and sterols)6 which possess potent anti-inflammatory and antioxidant properties.7 The possibility that unsaponifiable lipids from shea butter assist the skin in repairing a damaged skin barrier with adequate lipids is recognized in literature.8 This skin cream is employed as posttreatment to substitute the hydrolipidic film removed by the application of the TCA peel.
The serum used in this study was dispensed with the aim of maximizing dermal stimulation. It has been well established that products containing alpha-hydroxy acids induce cell renewal, and that this effect is correlated to the pH level of the formulation administered.9 Moreover, an acidic pH provides a favorable environment for proteolytic processing of POMC by enzyme PC1.10 It can be therefore argued that continued skin acidification in the days following application of the TCA peel might lead to a more efficient expression of the regenerative process.11,12
Photography. Photographs were taken on before and after treatment on Day 0, before treatment on Day 21, and before treatment on Day 51 using a VISIA-CR Facial Imaging System (Canfield Scientific, Parsippany, New Jersey). Photographs were full face, right side (45 degrees), and left side (45 degrees).
Efficacy. Investigator Facial Aging Assessments (Glogau) and Investigator Global Assessments (IGA, full face) were conducted at each visit and graded according to the scales in Table 1.
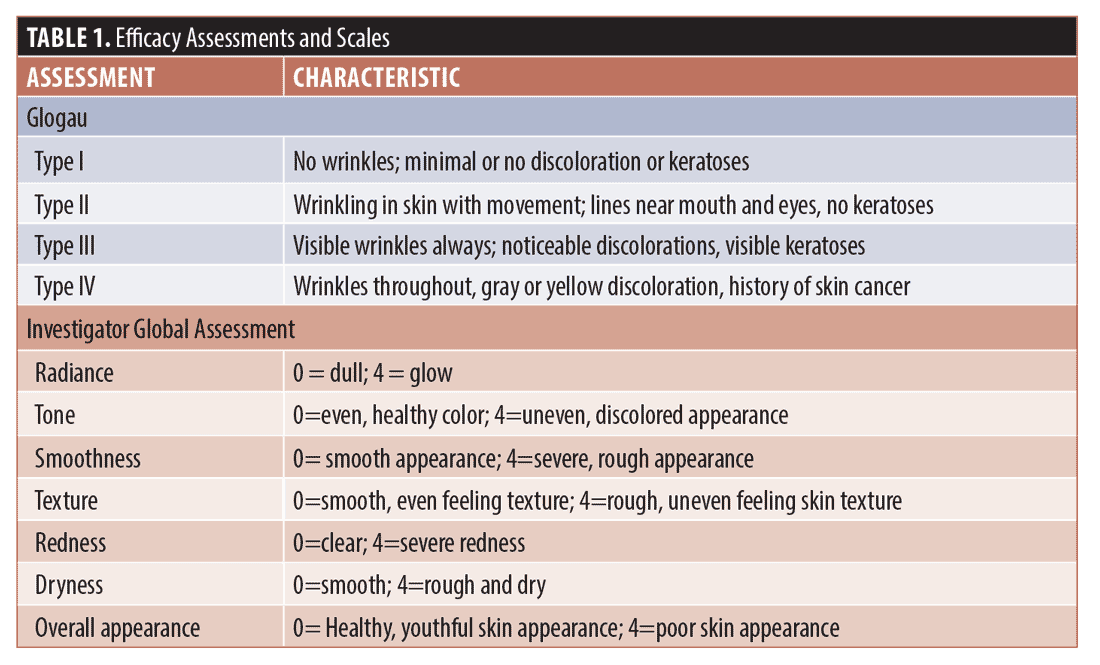
Safety. Investigator Objective Tolerability (IOTA, full face), and Subjective Tolerability (SOT, full face) assessments were conducted at every visit and graded (0=none, 1=minimal, 3=moderate, 4=severe). IOTA parameters were erythema, edema, dryness, and peeling and SOT parameters were stinging, tingling, dryness, and burning.
Data analysis. Since data consisted of small whole numbers over a narrow range, data were analyzed by non-parametric statistics with p<0.05 as the cutoff level for significance.
Results
Efficacy. The Friedman test, a non-parametric alternative to analysis of variance (ANOVA), was used to test for significance among repeated measurements for the Glogau, IGA, and tolerability scores. The median and range (maximum–minimum) values for each are shown in Table 2 for each visit. For the Glogau system, the median scores remained at 3.0 until Visit 4, when the median decreased to 2.0, which was non-significant when compared to baseline. Among IGA attributes, differences from baseline were significant among visits for tone (p=0.0174), smoothness (p=0.0014), texture (p=0.0017), redness, (p=0.0013) and overall appearance (p=0.0073), while the difference approached significance for radiance (p=0.0749). Lower median values denoted improvement for all attributes except radiance, for which a higher score indicated more glow in the skin. In each case the median scores began to decrease at Visit 2, 14 days after the initial treatment with the TCA-peroxide product, and the decrease continued through Visit 4, after the final treatment. Dryness was graded low and did not change throughout the study.
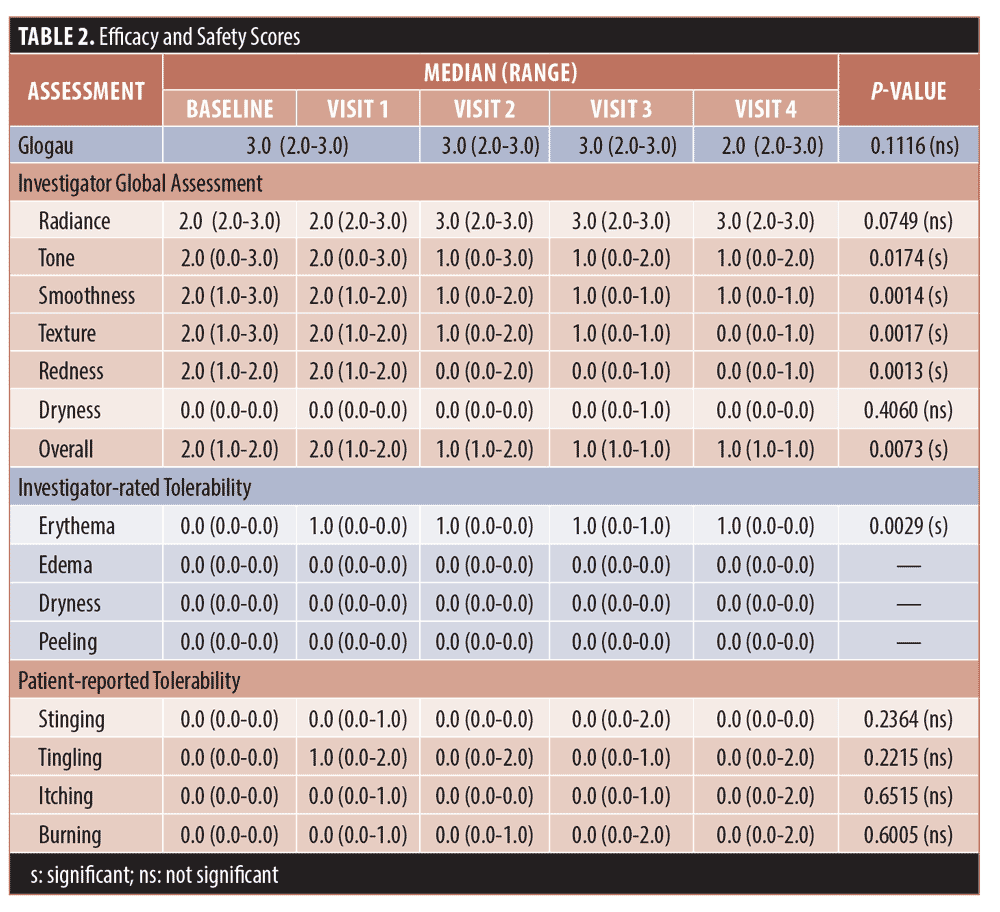
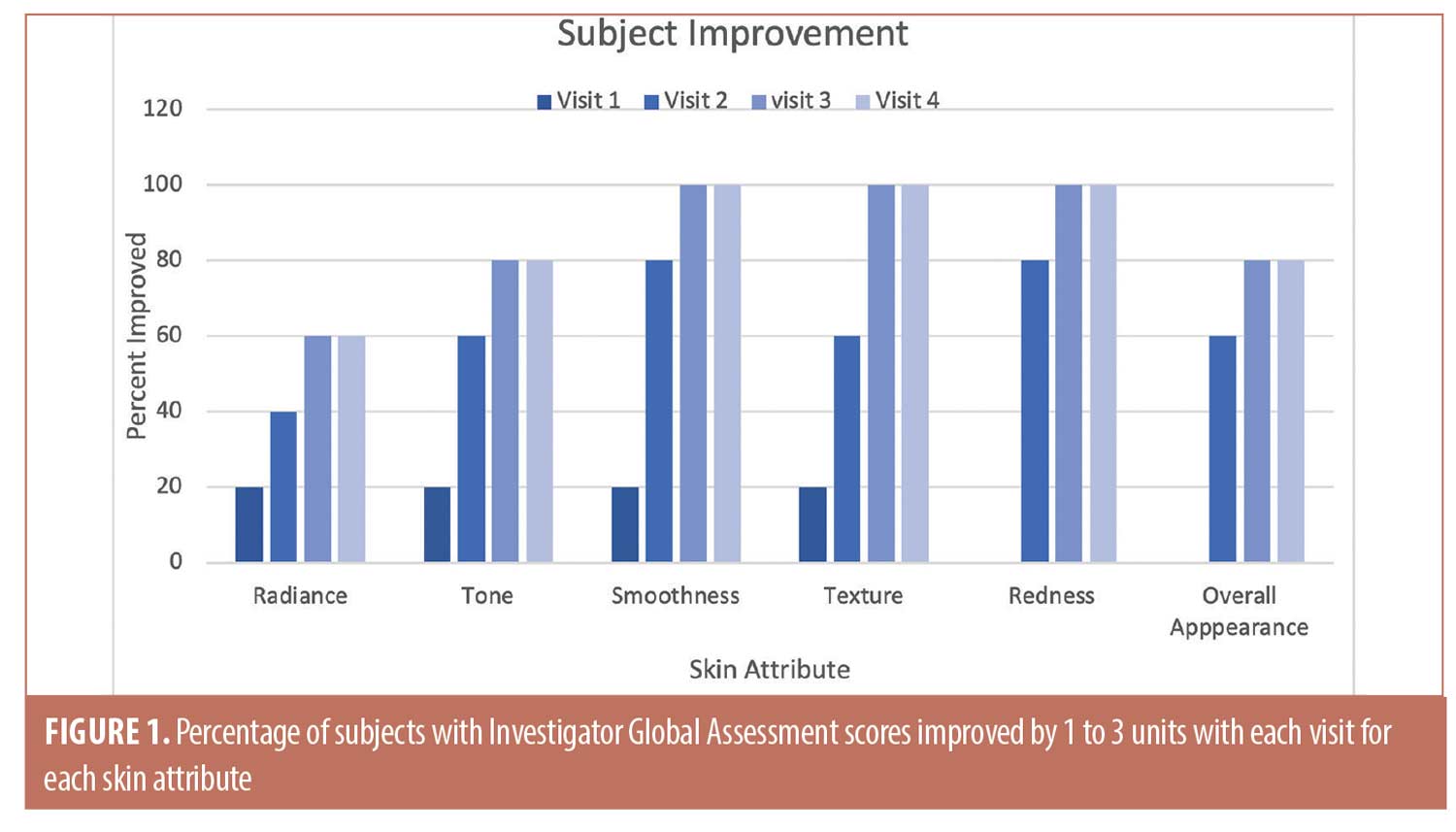
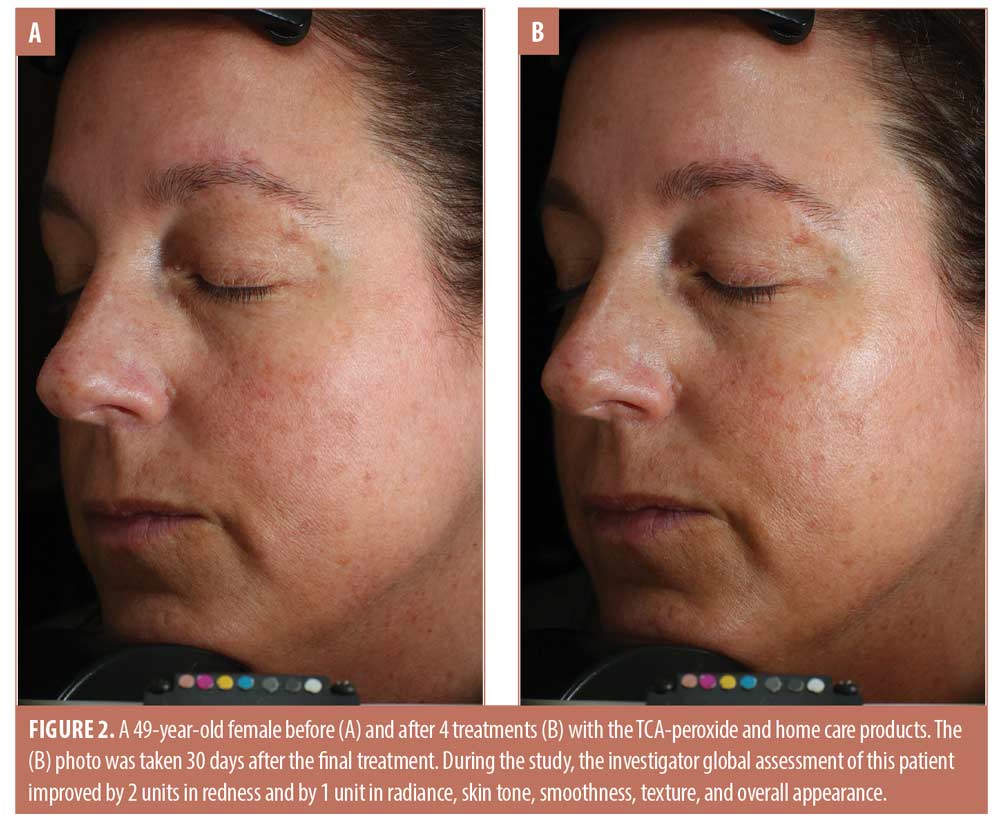
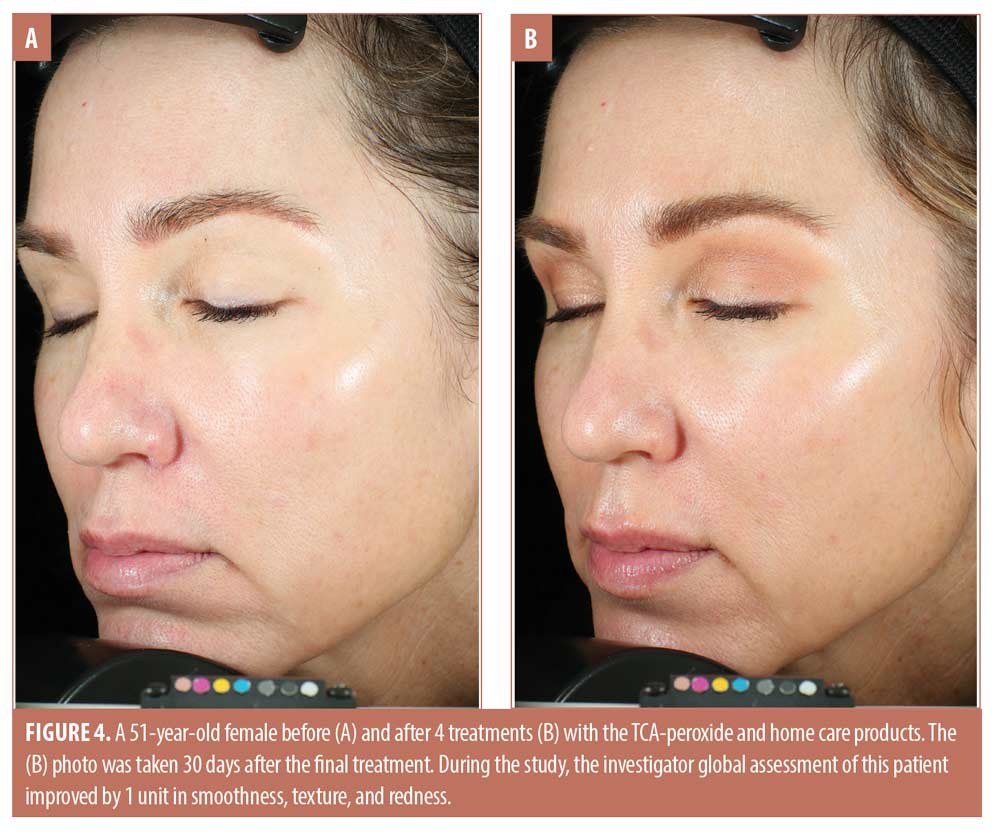
The trends toward increasing improvement with treatment are presented graphically in Figure 1, which shows the percentage of subjects that improved by 1 to 3 units compared to baseline for each IGA skin attribute. Twenty percent of subjects showed immediate improvement in radiance, tone, smoothness, and texture (Visit 1, immediately after treatment) while 100 percent of subjects showed improvement by Visit 3 for smoothness, texture, and redness and the improvements persisted to the end of the study. Clinical examples are shown in Figures 2 to 4.
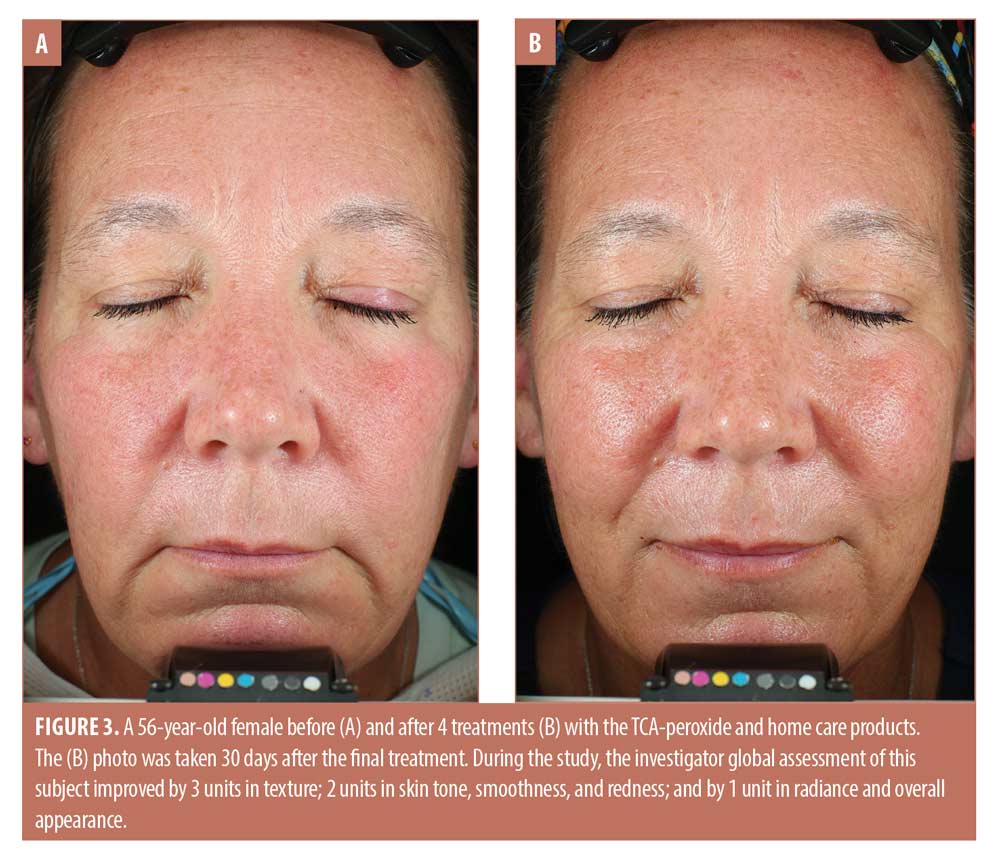
Safety. As shown in Table 2, investigator tolerability assessments showed a slight increase (0.0 to 1.0) for erythema, which was statistically but not clinically significant. Assessments scores were 0.0 throughout the study for edema, dryness, and peeling, and were not analyzed statistically.
Subjective tolerability assessments showed median scores of 0.0 for stinging, tingling, itching, and burning for all visits except for the 1.0 median value at Visit 2 for tingling. Range was variable for tingling, itching, and burning toward the end of the study. Changes from baseline were not significant for any assessment.
Discussion
The results of our study show that continued treatment with the TCA-peroxide peel resulted in rapid and persistent improvement for most IGA skin attributes throughout the 51-day study. The product was well-tolerated and adverse effects were not observed.
Adverse effects were limited to transient tingling and mild erythema after each treatment. Recent reviews have reported that complications of chemical peels included skin edema, burning and itching sensations, blistering, infections, scarring, and prolonged erythema.13,14 Acneiform eruptions and lines of demarcation between treated and non-treated areas have also been reported.1 These side effects were not observed in our study.
The inclusion of hydrogen peroxide in the TCA product was predicated on its action as a reactive oxygen species. At low concentrations, reactive oxygen species may act as cellular messengers in cell proliferation, angiogenesis, extracellular matrix synthesis, and other events in wound repair.15 Low doses of hydrogen peroxide have mitogenic effects, can mimic the function of growth factors, and can diffuse freely through cell membranes.16,17 Topically applied hydrogen peroxide (0.15%) also facilitates wound closure.17
The demonstrated efficacy of the TCA-peroxide product may be explained by its influence on the skin stress response system (SSRS) proposed by Slominski et al.3 The SSRS can be understood by first reviewing the hypothalamic-pituitary-adrenal (HPA) axis for systemic stress. In the HPA axis, the hypothalamus releases corticotropin releasing hormone (CRH), the pituitary CRH receptors (CRH-R) are activated, and proopiomelanocortin (POMC)-derived peptides are released in response to systemic stress.3,4 In 1999, Slominski et al3 provided evidence that skin cells also produce CRH and express functional CRH-R1, suggesting that in mammalian skin, a local CRH/CRH-R neuroendocrine pathway, or “skin stress response system,” exists that may be activated in response to local stressors or proinflammatory cytokines. Once activated, the SSRS would limit tissue damage and restore homeostasis.3,4 Kimura et al,18 working with murine and human skin, provided evidence that TCA activates the SSRS by inducing keratinocytes to produce POMC and melanocortin receptor 1 without dependence on hypothalamic CRH, and that the biological effects of POMC itself are responsible for the activation of the epidermal SSRS by TCA.
TCA at 35% to 50% is considered a medium chemical peel that may be used to treat mild to moderate photoaging.14 Medium-depth peels penetrate the papillary dermis to the upper reticular dermis at a depth of 450 Mu-m. When peels affect the papillary or upper to mid-reticular dermis, they help to reduce rhytids and promote deposition of elastin and collagen.13,19,20
The concentration of TCA in the TCA-peroxide product is 33%; however, subjects did not experience white frosting and subsequent shedding of the epidermal layers as with traditional TCA peels. This allows for less downtime and a lower risk of side effects, such as postinflammatory hyperpigmentation or infections.
Despite the availability of lasers and energy-based modalities, chemical peels remain a rapid, safe, and cost-effective technique for skin rejuvenation, particularly in an aging, photodamaged population.14
Limitations. Limitations of the present study are the small number of subjects and short follow-up time. The encouraging results of the present study justify a future study with more subjects, control subjects, and longer follow-up times to establish maintenance treatment.
Conclusion
The TCA-peroxide and home care products used in this study appear to provide rapid and consistent improvement of photodamaged skin without adverse effects.
Acknowledgments
The authors thank Fred Wilson of WilWrite (Camillus, New York) for statistical analysis and assistance in the preparation of this manuscript.
References
- Rendon MI, Berson DS, Cohen JL, et al. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3: 32–43.
- Fischer TC, Perosino E, Poli F, et al. Cosmetic Dermatology European Expert Group. Chemical peels in aesthetic dermatology: an update 2009. J Eur Acad Dermatol Venereol. 2010;24:281–292.
- Slominski AT, Botchkarev V, Choudhry M, et al. Cutaneous expression of CRH and CRH-R. Is there a “skin stress response system?” Ann N Y Acad Sci. 1999;885:287–311.
- Kimura A, Kanazawa N, Li HJ, et al. Influence of chemical peeling on the skin stress response system. Exp Dermatol. 2012;21 Suppl 1:8–10.
- Castellana R, De Sa Viana AC, Rizzi L. Fibroblast stimulation through the activation of the endocrine system for the application of trichloroacetic acid. La Medicina Estetica. 2013;3:188–193.
- Megnanou RM, Niamke S. Improving the optimized shea butter quality: a great potential of utilization for common consumers and industrials. SpringerPlus. 2015;4:667–776.
- Lin TK, Zhong L, Santiago JL. Anti-Inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19:70–90.
- Lodén M, Andersson AC. Effect of topically applied lipids on surfactant-irritated skin. Br J Dermatol. 1996;134:215–220.
- Smith WP. Hydroxy acids and skin aging. Cosmet Toilet. 1994;109:41–48.
- Tanaka S, Yora T, Nakayama K, et al. Proteolytic processing of pro-opiomelanocortin occurs in acidifying secretory granules of AtT-20 cells. J Histochem Cytochem. 1997;45:425–436.
- Harno E, Gali Ramamoorthy T, Coll AP, White A. POMC: The physiological power of hormone processing. Physiol Rev. 2018;98:2381–2430.
- Cawley NX, Li Z, Loh YP. 60 years of POMC: Biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J Mol Endocrinol. 2016;56(4):T77–T97.
- Samargandy S, Raggio BS. Skin Resurfacing Chemical Peels. In: StatPearls. StatPearls Publishing, Treasure Island (FL); 2020.
- O’Connor AA, Lowe PM, Shumack S, Lim AC. Chemical peels: A review of current practice. Australas J Dermatol. 2018;59:171–181.
- Khanna S, Wallace W. Wound healing: oxygen and emerging therapeutics. Antioxid Redox Signal. 2002; 4: 961–963.
- Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3(12):1129–1134.
- Roy S, Khanna S, Nallu K, et al. Dermal wound healing is subject to redox control. Mol Ther. 2006;13:211–220.
- Kimura A, Kanazawa N, Li HJ, et al. Influence of trichloroacetic acid peeling on the skin stress response system. J Dermatol. 2011;38:740–747.
- Weissler JM, Carney MJ, Carreras Tartak JA, et al. The evolution of chemical peeling and modern-day applications. Plast Reconstr Surg. 2017;140:920–929.
- Truchuelo M, Cerdá P, Fernández LF. Chemical peeling: a useful tool in the office. Actas Dermosifiliogr. 2017;108:315–322.

