 J Clin Aesthet Dermatol. 2020;13(5):38–43
J Clin Aesthet Dermatol. 2020;13(5):38–43
by Jason Solway, DO; Michael McBride, DO; Furqan Haq, PhD, MPH; Waheed Abdul, MD; and Richard Miller, DO
Drs. Solway, Haq, Abdul, and Miller are with Largo Medical Center in Largo, Florida. Dr. McBride is with Riverside Methodist Hospital in Columbus, Ohio.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Previous studies have demonstrated that a whole-food, plant-based (WFPB) diet can aid in the prevention, and in some cases reversal, of some of the leading chronic diseases in the United States. The medical literature on the relationship between diet and disease is steadily growing. Over the last decade, the possible connection between diet and many dermatological conditions has been studied, including skin aging.
Objective. As patients are increasingly seeking dietary advice from their dermatologist related to preventing and reversing the aging of skin, dermatologists need an evidence-based approach to tackle this challenging topic. This review focuses on dietary factors that contribute to telomere length, a marker for cellular aging. Although various factors contribute to accelerating telomere shortening, this review focuses on dietary factors that contribute to telomere length, specifically gerontotoxins and antioxidants. These can be measured in the blood, making them biomarkers of accelerated cellular skin aging. Included in this discussion is an evidence-based approach to increase the amount of antioxidants and decrease the amount of gerontotoxins in the diet, resulting in healthier skin.
Methods. A comprehensive MEDLINE (PubMed) literature review search was performed. Keywords used included: WFPB, telomerase, coronary artery disease, cellular aging, cigarette smoke, photoaging, telomeres, antioxidants, gerontotoxins, intrinsic cutaneous aging, extrinsic cutaneous aging, advanced glycation end products (AGEs), vitamin E, vitamin C, vitamin E, CoQ10, polyphenols, chlorophyll, zeaxanthin, polyunsaturated fatty acids, eicosapentaenoic acid, docosahexaenoic acid, alpha-linolenic acid, and monounsaturated fatty acids. Inclusion criteria included the above stated keywords and access to full text.
Results. A WFPB diet maximizes the antioxidant potential within our cells by providing essential vitamins, including vitamins A, C, and E. It also helps to eliminate harmful carcinogens and gerontotoxins within our bloodstream and has been shown to lengthen telomeres, which prevents cellular damage.
Conclusion. Evidence obtained within this literature review supports a WFPB diet for preventing skin aging. .
Keywords: Diet, dermatology, whole-food, plant-based, antioxidants, gerontotoxins, aging skin
Kaiser Permanente defines a whole-food, plant-based (WFPB) diet as, “an eating plan that includes lots of plant foods in their whole, unprocessed form, such as vegetables, fruits, beans, lentils, nuts, seeds, whole grains, and small amounts of healthy fats. It does not include animal products, such as red meat, poultry, fish, dairy, or eggs. It also does not include processed foods or sweets.”1
A WFPB diet is often compared to a vegan diet. However, there are important differences that should be noted. Some individuals might equate the word vegan with healthy. However, vegan diets might include processed oils, sugars, and white flour and not include fresh vegetables or fruits. Veganism excludes animal products in the diet, but does not automatically indicate a diet rich in antioxidants. A vegan diet specifies what the diet does not contain, but does not specify what it does contain.2 In contrast, a WFPB diet indicates a diet abundant in antioxidant-rich plant foods while avoiding processed oils, sugars, and animal products. Eating a WFPB diet can provide the nutrients, vitamins, and minerals necessary to maintain younger cells and excludes the high amount of saturated fat obtained from animal products, which damages cells.3,4
A WFPB diet has been demonstrated to lengthen telomeres and reverse the aging process of deoxyribonucleic acid (DNA).5–7 It is also responsible for preventing and reversing the leading chronic diseases in America, specifically coronary artery disease (CAD).8–11 Additionally, WFPB diet has been shown to reduce the amount of gerontotoxins measured in the blood, making them biomarkers of accelerated cellular skin aging, as well as increase the amount of antioxidants, which ultimately can translate into healthier and more youthful skin. Although various factors contribute to accelerated telomere shortening, this review focuses on dietary factors that contribute to telomere length, specifically gerontotoxins and antioxidants.12,13
Methods
A comprehensive MEDLINE (PubMed) literature review search was performed. Keywords used included: WFPB, telomerase, coronary artery disease, cellular aging, cigarette smoke, photoaging, telomeres, antioxidants, gerontotoxins, intrinsic cutaneous aging, extrinsic cutaneous aging, advanced glycation end products (AGEs), vitamin E, vitamin C, vitamin E, CoQ10, polyphenols, chlorophyll, zeaxanthin, polyunsaturated fatty acids, eicosapentaenoic acid, docosahexaenoic acid, alpha-linolenic acid, and monounsaturated fatty acids. Inclusion criteria included the above stated keywords and access to full text. A total of 79 articles were identified for inclusion in our review.
Telomerase and the Cellular Aging Process: Preventing and Reversing Chronic Disease
To successfully articulate the importance of an evidence-based approach when choosing a healthy diet for our skin, understanding the role that telomerase plays in the aging process of the body, as a whole, must be established. Inside human cells, there are 46 strands of DNA coiled into chromosomes. Telomeres are located at the tip of each chromosome and keep DNA from unraveling and fraying. The analogy of plastic tips at the end of shoelaces has been used to explain the position of telomeres.14 Each time DNA replicates, telomeres are shortened. Once telomeres are completely gone, the cell dies.15
Telomerase is the enzyme in human cells responsible for rebuilding telomeres. Multiple factors influence the activity of telomerase. In a pilot study, Ornish et al6 demonstrated that lifestyle and diet modifications influenced telomerase activity to slow the aging process and possibly reverse it. This was evident in the increased length of telomeres observed in the five-year follow-up study. The control group, who did not change their lifestyle, had telomeres that shrank with age. The intervention group, who consumed a WFPB diet, had telomeres that appeared to increase in length.5–7
Several studies have reported the potential of a WFPB diet to prevent and reverse pre-existing heart disease.8–11 One of these studies by Esseltyn et al8 demonstrated diet modifications and cholesterol-lowering drugs could treat the symptoms of cardiovascular disease, specifically angina, graded on the Canadian Cardiovascular Society Scale. and prevent clinical progression. Another study by Ornish et al11 demonstrated that lifestyle changes, such as diet modifications, moderate aerobic exercise, stress management training, smoking cessation, and group psychosocial support, prevented the progression of coronary artery disease and decreased existing coronary disease, compared to the control group.
In both studies, dietary modifications included less than 10 percent of its calories from fat and avoiding most animal products in exchange for a predominantly WFPB diet.8 Together, these reports suggest that a diet that is limited in animal products and rich in grains, legumes, vegetables, and fruits has the potential to decrease the systemic inflammatory process associated with cardiovascular disease and reverse the age of blood vessels. More broadly, these initial discoveries have paved the way for further research related to preventing and reversing other age-related concerns, such as the symptoms of skin aging.
Inflammation and Skin Aging
Many common dermatologic conditions, including accelerated skin aging, stem from an inflammatory process. Similar to heart disease, the human skin experiences the typical intrinsic aging process through genetically determined loss of cell function with age.5 In addition to this intrinsic process, extrinsic factors contribute to skin aging, including exposure to ultraviolet (UV) irradiation, smoking, and pollution, as well as sleep disturbance and poor nutrition.16–19 Both intrinsic and extrinsic inflammatory processes combine to manifest in the skin aging as fine wrinkles, loss of elasticity, dryness, and sallowness.18 This skin aging process has greater implications aside from a cosmetically poor appearance. The aging process negatively affects skin permeability, angiogenesis, lipid and sweat production, immune function, and vitamin D synthesis. The ultimate consequence of these processes manifests as impaired wound healing, skin atrophy, vulnerability to external stimuli, and even the development of benign and malignant pathological processes.19
Gerontotoxins. Gerontotoxins, a contributor of systemic inflammation, are a group of toxins that cause our cells to age. A well-studied toxin is an advanced glycation end-product (AGE).20 Glycation is a nonenzymatic chemical process that involves the formation of a covalent bond between a sugar molecule (i.e., glucose, fructose) and a protein or lipid. This differs from physiologic glycation, which is under enzymatic control.21 AGEs accumulating within the skin can cause a rapid stiffening of collagen, elastin, vitronectin, and laminin. This can present clinically as skin ulcers and delayed skin healing. This process of uncontrolled glycation also reduces the cell’s ability to generate nitric oxide from L-arginine, which is required for proper cross-linking of collagen fibers, and inactivates proteins responsible for collagen and elastin repair.22 Clinically, this results in lower skin tensile strength, as seen in the aged skin of older individuals.24
AGEs accumulate within the body from both endogenous and exogenous sources at a rate reported to be around 3.7 percent annually.21 External factors, including UV irradiation, cigarette smoking, poor dietary sources, and certain cooking methods accelerates the rate of AGE formation and strengthens the covalent bond formed.24,25 Uribarri et al12,13 measured the AGE units in more than 500 foods. The findings showed meat and processed foods contained the highest number of AGE units, while WFPB products contained the least. Additionally, cooking foods using high, dry heat (e.g., roasting, grilling) appeared to increase their AGE content.
The Role of Antioxidants in Skin Health
In addition to AGEs, oxidative damage appears to accelerate the skin aging process. Multiple chemical reactions occur within the skin, prompting the development of reactive oxygen species (ROS) and oxidative damage.26 ROS are neutralized through a cascade of reactions by the skin’s innate defense mechanism. Antioxidants exist to neutralize and mitigate ROS through the donation of electrons. The primary antioxidant found in the skin is vitamin E. After acting as an antioxidant, the oxidized form of vitamin E needs to be regenerated. Vitamin C completes this process. Vitamin C is considered a secondary antioxidant in the skin and must be replenished from either a dietary source or a tertiary antioxidant, such as vitamin A. This cascade of electron donations ultimately helps to reduce the oxidative damage from ROS.26 On average, plant foods contain 64 times more antioxidants than animal products.26
Due to their plant pigments, green vegetables contain the highest amount of antioxidants of any vegetables and berries contain the highest amount of antioxidants of any fruit. Darker colors in plant food translates to more antioxidants.27,28 Regular consumption of antioxidant-rich foods can prevent the circulation of oxidized fats in the bloodstream, which can damage the sensitive walls of small blood vessels.29
Vitamin E: the primary antioxidant. The vitamin E complex is required to maintain proper skin health. This complex consists of eight compounds called tocopherols.30 Constant consumption of tocopherolic sources is needed to ensure sufficient systemic levels of vitamin E. Without adequate stores, lipid peroxidation and collagen cross-linking occurs in the skin and accelerates skin aging.31 The most biologically active source of vitamin E in the skin, alpha-tocopherol, functions to terminate lipid radical chain reactions, stabilizing cell membranes against damage by phospholipase A, free fatty acids, and lysophospholipids.32 Vitamin C must be present within the skin to regenerate the antioxidant properties of vitamin E. These two vitamins act synergistically to prevent damage from oxidative stress. In addition to vitamin C, both coenzyme Q10 (CoQ10) and glutathione can recycle the oxidized form of vitamin E, albeit to a lesser degree.30,33
Vitamin C: a secondary antioxidant. Vitamin C, also known as L-ascorbic acid, works in the skin as an antioxidant by scavenging and quenching free radicals to regenerate vitamin E from its radical form.35,36 Vitamin C promotes gene expression activity for collagen production and acts as a cofactor for proline and lysine hydroxylase enzymes. These two enzymes stabilize the tertiary molecule of collagen and have been shown to play an important role in wound healing.35–43 In addition to acting as a secondary antioxidant, vitamin C is thought to promote fibroblast proliferation, migration, and replication of the base excision repair of potentially mutagenic DNA lesions.44 This characteristic could reverse or prevent skin cancers and enhance wound repair.45 These properties make vitamin C a necessary component to prevent skin damage.
Vitamin A: a tertiary antioxidant. Vitamin A acts as a tertiary antioxidant within the skin by quenching singlet oxygen species. A skin receptor for vitamin A confirms this crucial aspect for proper skin health.46 Vitamin A is mainly stored in the liver, and an adequate level is necessary to prevent the chemical carcinogens in the epithelial tissues of the bronchi, trachea, stomach, uterus, and skin.47,48 Carotenoids are vitamin A derivatives that are commonly used in topical antiaging formulations associated with the prescription retinoid tretinoin. Their topical photoprotective roles have been well-established, but less is known about the effects from their ingested forms.46
Chlorophyll and CoQ10. Carcinogens can damage DNA, causing cellular aging. Chlorophyll, the most ubiquitous plant pigment in the world and responsible for giving plants their green color, has been found to block damage to DNA from carcinogens. Chlorophyllin (CHL) is a water-soluble derivative of chlorophyll. Specifically, CHL protects cells against mutagenic effects of benzopyrene, cyclophosphamide, heterocyclic amines, aflatoxin, heavy metals, and ionizing radiation. Because the diverse mutagens neutralized by CHL, multiple mechanisms have been hypothesized on how it occurs. One mechanism includes the direct antioxidant properties of CHL. Another mechanism is CHL’s ability to form complexes with aromatic mutagens. The complexes are thought to be maintained via stacking (pi-pi) interactions between the flat aromatic molecules of the mutagen and the porphyrin rings of CHL. Thus, CHL captures the mutagens and prevents them from uptake into the cells and interactions with DNA.49–51
In one human study from Jubert et al,52 volunteers drank a solution containing an Institutional Review Board-approved dose of a carcinogen (14C-AFB1) with or without an amount of chlorophyll equal to what is found in six cups of spinach. Three different protocols were used for consumption. For Protocol 1, fasted subjects received 30ng by capsule followed with 100mL water, followed by normal eating and drinking after two hours. Blood and cumulative urine samples were collected over 72 hours, and 14C-AFB1 equivalents were determined by accelerator mass spectrometry. Protocols 2 and 3 were similar except capsules also contained 150mg of purified chlorophyll or chlorophyllin, respectively. The study revealed rapid human AFB1 uptake and urinary elimination (95% complete by 24 hours) kinetics. Chlorophyll and chlorophyllin treatment each significantly impeded AFB1 absorption and reduced Cmax and AUCs (plasma and urine) in one or more subjects. These initial results provide AFB1 pharmocokinetic parameters previously unavailable for humans, and suggest that chlorophyll and chlorophyllin co-consumption might limit the bioavailability of ingested aflatoxin in humans, as they do in animal models.
Chlorophyll also plays a role in regenerating CoQ10, also known as ubiquinol. CoQ10 is an endogenously produced compound that acts as an antioxidant by scavenging free radicals, protecting our cells from DNA damage. This reaction oxidizes ubiquinol to ubiquinone. Ubiquinol must be regenerated from ubiquinone to become effective again. This reaction requires both chlorophyll and sunlight acting synergistically.53,54 A study by Zmitek et al55 found dietary supplementation containing CoQ10 over a 12-week period exhibited several antiaging effects, including reduced skin wrinkles, increased skin firmness, and improvments in skin smoothness and microrelief.56–58
Polyphenols. Polyphenols have gained popularity in recent years due to their antioxidant properties and potential prevention of certain cancers and cardiovascular and neurodegenerative diseases.59 They are commonly found in fruits and plant-derived beverages, such as fruit juices, tea, coffee, red wine, vegetables, cereals, chocolate, and dry legumes.30,60 Studies observing polyphenols combined with sunscreen on animal models showed prevention from UV-induced skin damage, oxidative stress, and DNA damage.61 One study from Yoon et al62 found that a 24-week course of daily cocoa flavanol consumption, which is high in polyphenols, improved skin wrinkles and elasticity in women with moderate skin photoaging.
Zeaxanthin. Multiple studies have reported the beneficial effects of topical application and oral ingestion of zeaxanthin-based formulations, including its isomer lutein, as an antioxidant to prevent skin wrinkles and improve skin hydration.63–67
The Role of Fatty Acids in Protecting Against Skin Aging
Polyunsaturated fatty acids (PUFAs). PUFAs—in particular, eicosapentaenoic (EPA) and docosahexaenoic acids (DHAs)—modulate and reduce skin inflammation.68 Several studies have reported the association between a high dietary intake of EPA and DHA and protection from both UVB-induced damage and early genotoxic markers of human skin cancers.69–71
Alpha-linolenic acid (ALA) is an essential fatty acid that acts as the precursor molecule of the n-3 PUFA family. ALA cannot be synthesized endogenously and must be consumed from dietary sources. Once ingested, ALA is converted to other n-3 PUFAs. EPA and DHA are two n-3 PUFAs within the body that are derived from the conversion of dietary ALA. Dietary sources account for the majority of the body’s store of n-3 PUFAs.72
A higher dietary intake of ALA reduces senile dryness and skin atrophy in middle-aged women by acting as a source for EPA and DHA synthesis.72 A large study showed an inverse association between the severity of skin aging and ALA intake in men and between the severity of skin aging and EPA intake in women, independent of environmental factors, such as smoking and UV irradiation, which are known to play a role in the skin aging process.68 In addition, a low intake of ALA has been associated with dermatitis, further suggesting a significant role in the skin-aging process.73–75
Monosaturated fatty acids (MUFAs). In addition to the beneficial role that PUFAs play in the prevention of skin aging, multiple reports have suggested a similar beneficial role of MUFAs. By reducing oxidative stress, insulin resistance, and related inflammation, MUFAs potentially play a major role in the prevention of skin aging.68,75–79 Latreille et al78 observed a significant association between total intake of MUFA and skin aging in men but not in women.Elsewhere, a higher dietary intake of olive oil was inversely correlated with the severity of skin photoaging, supporting the beneficial role of olive oil, which is high in MUFAs, in preventing severe skin aging.79
Summary and Dietary Recommendations
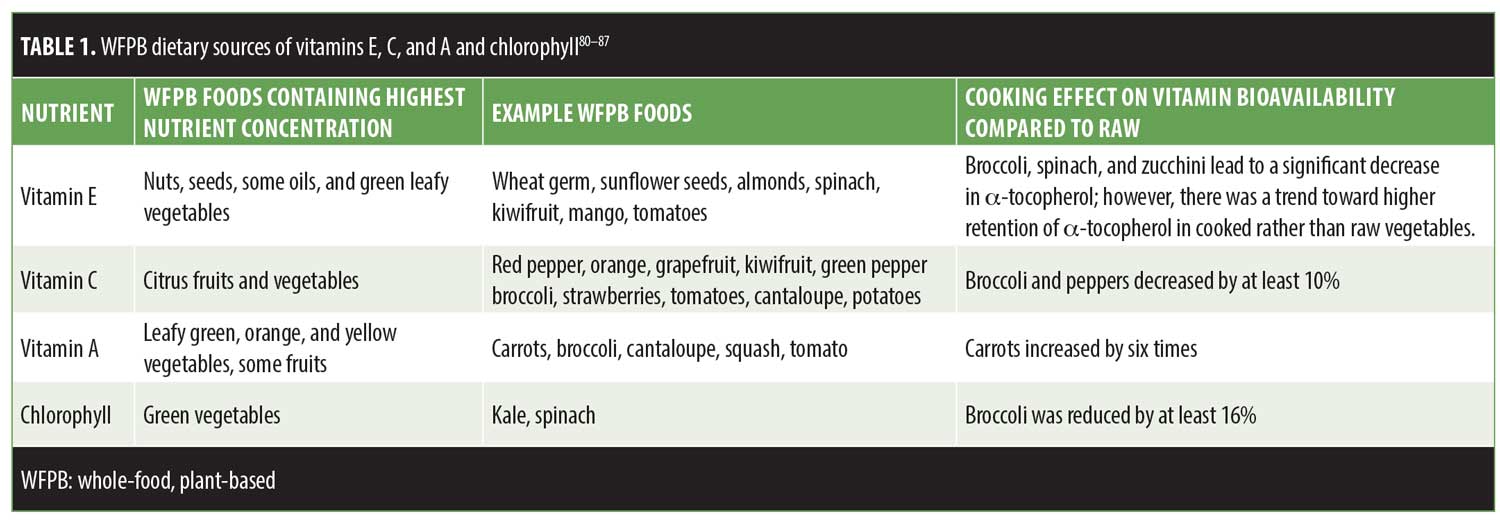
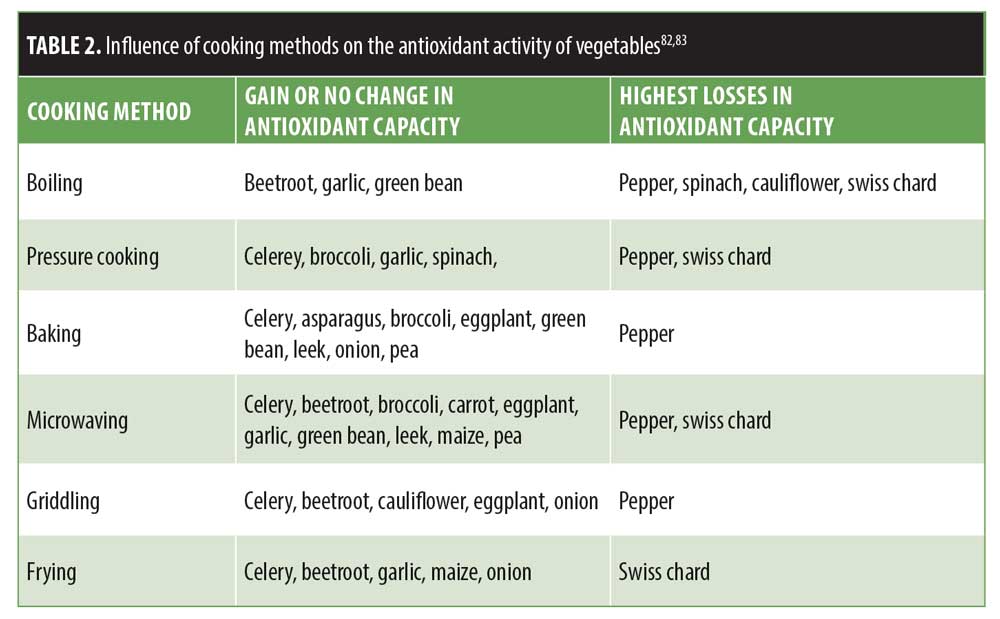
In conclusion, when patients inquire about a diet that might contribute to younger-looking skin, evidence supports the recommendation to follow a WFPB diet. Demonstrated to lengthen telomeres, a marker for cellular aging, a WFPB diet has been shown to not only maximize the antioxidant potential of our cells, but also eliminate harmful carcinogens and gerontotoxins entering our bloodstream. Studies investigating these protective characteristics of the nutrients consumed in abundance in a WFPB diet support the diet’s potential to contribute to healthier, younger-looking skin. Tables 1 and 2 provide evidence-based recommendations for dietary sources of antioxidants and cooking methods to maximize antioxidant potential.83,84 Additional research on the effects of a WFPB diet on skin health will enhance our understanding of the role nutrition plays in the aging process and our overall health.
Limitations. This review paper has several limitations. The references identified were limited to MEDLINE (PubMed). Also, multiple studies referenced had a small sample size of participants. Some studies used were unblinded and others used animals as subjects. Although we found a correlation between WFPB and their innate properties, no literature directly studied the subjects with WFPB diet and objective skin aging measures.
References
- Permanente K. The plant-based diet. Available at: https://thrive.kaiserpermanente.org/care-near-you/northern-california/santarosa/wp-content/uploads/sites/15/2015/09/New-Plant-Based-Booklet-1214_tcm28-781815.pdf. Accessed June 17, 2019.
- Merriam-Webster Inc. The Merriam-Webster Dictionary. Springfield, MA: Merriam-Webster, Inc.; 2016.
- Pritikin R. The New Pritikin Program. New York, NY: Simon and Shuster; 1990.
- Temple NJ, Burkitt DP. Western Diseases: Their Dietary Prevention and Reversability. Totowa, NJ: Humana Press; 1994.
- Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14(11):1112–1120.
- Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048–1057.
- Skordalakes E. Telomerase and the benefits of healthy living. Lancet Oncol. 2008;9(11):1023–1104.
- Esselstyn CB, Ellis SG, Medendorp SV, Crowe TD. A strategy to arrest and reverse coronary artery disease: a 5-year longitudinal study of a single physician’s practice. J Fam Pract. 1995;41(6): 560–568.
- Morrison LM. A nutritional program for prolongation of life in coronary atherosclerosis. J Am Med Assoc. 1955;159(15):1425–1428.
- Morrison LM. Diet in coronary atherosclerosis. J Am Med Assoc. 1960;173:884–888.
- Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280(23):2001–2007.
- Uribarri J, Cai W, Sandu O, et al. Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann N Y Acad Sci. 2005;1043:461–466.
- Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911–916.e12.
- Greger M, Stone G. How not to die: discover the foods scientifically proven to prevent and reverse disease. London: Pan Books; 2018.
- Jiang H, Ju Z, Rudolph KL. Telomere shortening and ageing. Z Gerontol Geriatr. 2007;40(5):314–324.
- Bernhard D, Moser C, Backovic A, Wick G. Cigarette smoke—an aging accelerator? Exp Gerontol. 2007;42(3):160–165.
- Fisher GJ, Kang S, Varani J, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138(11):1462–1470.
- Makrantonaki E, Zouboulis CC. Molecular mechanisms of skin aging: state of the art. Ann N Y Acad Sci. 2007;1119:40–50.
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29(1):3–14.
- Rahmadi A, Steiner N, Munch G. Advanced glycation endproducts as gerontotoxins and biomarkers for carbonyl-based degenerative processes in Alzheimer’s disease. Clin Chem Lab Med. 2011;49(3):385–391.
- Corstjens H, Dicanio D, Muizzuddin N, et al. Glycation associated skin autofluorescence and skin elasticity are related to chronological age and body mass index of healthy subjects. Exp Gerontol. 2008;43(7):663–667.
- Danby FW. Nutrition and aging skin: sugar and glycation. Clin Dermatol. 2010;28(4):409–411.
- Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114(6):597–605.
- Cerami C, Founds H, Nicholl I, et al. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci U S A. 1997;94(25):13915–13920.
- Yamauchi M, Prisayanh P, Haque Z, Woodley DT. Collagen cross-linking in sun-exposed and unexposed sites of aged human skin. J Invest Dermatol. 1991;97(5):938–941.
- Carlsen MH, Halvorsen BL, Holte K, et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. 2010;9:3.
- Hernandez-Marin E, Galano A, Martinez A. Cis carotenoids: colorful molecules and free radical quenchers. J Phys Chem B. 2013;117(15): 4050–4061.
- McCullough ML, Peterson JJ, Patel R, et al. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr. 2012;95(2):454–464.
- Kelly PJ, Morrow JD, Ning M, et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: the Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke. 2008;39(1):100–104.
- Schagen SK, Zampeli VA, Makrantonaki E, Zouboulis CC. Discovering the link between nutrition and skin aging. Dermatoendocrinol. 2012;4(3):298–307.
- Igarashi A, Uzuka M, Nakajima K. The effects of vitamin E deficiency on rat skin. Br J Dermatol. 1989;121(1):43–49.
- Kagan VE. Tocopherol stabilizes membrane against phospholipase A, free fatty acids, and lysophospholipids. Ann N Y Acad Sci. 1989;570: 121–135.
- Beyer RE. The role of ascorbate in antioxidant protection of biomembranes: interaction with vitamin E and coenzyme Q. J Bioenerg Biomembr. 1994;26(4):349–358.
- Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol. 1993;71(9):725–731.
- Hinek A, Kim HJ, Wang Y, et al. Sodium L-ascorbate enhances elastic fibers deposition by fibroblasts from normal and pathologic human skin. J Dermatol Sci. 2014;75(3):173–182.
- Ivanov V, Ivanova S, Kalinovsky T, et al. Inhibition of collagen synthesis by select calcium and sodium channel blockers can be mitigated by ascorbic acid and ascorbyl palmitate. Am J Cardiovasc Dis. 2016;6(2):26–35.
- Kishimoto Y, Saito N, Kurita K, et al. Ascorbic acid enhances the expression of type 1 and type 4 collagen and SVCT2 in cultured human skin fibroblasts. Biochem Biophys Res Commun. 2013;430(2):579–584.
- Kivirikko KI, Myllyla R, Pihlajaniemi T. Protein hydroxylation: prolyl 4-hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit. FASEB J. 1989;3(5):1609–1617.
- May JM, Harrison FE. Role of vitamin C in the function of the vascular endothelium. Antioxid Redox Signal. 2013;19(17):2068–2083.
- May JM, Qu ZC. Transport and intracellular accumulation of vitamin C in endothelial cells: relevance to collagen synthesis. Arch Biochem Biophys. 2005;434(1):178–186.
- Miller RL, Elsas LJ, Priest RE. Ascorbate action on normal and mutant human lysyl hydroxylases from cultured dermal fibroblasts. J Invest Dermatol. 1979;72(5):241–247.
- Parsons KK, Maeda N, Yamauchi M, et al. Ascorbic acid-independent synthesis of collagen in mice. Am J Physiol Endocrinol Metab. 2006;290(6):
E1131–E1139. - Pihlajaniemi T, Myllyla R, Kivirikko KI. Prolyl 4-hydroxylase and its role in collagen synthesis. J Hepatol. 1991;13 Suppl 3:S2–S7.
- Duarte TL, Cooke MS, Jones GD. Gene expression profiling reveals new protective roles for vitamin C in human skin cells. Free Radic Biol Med. 2009;46(1):78–87.
- Catani MV, Savini I, Rossi A, et al. Biological role of vitamin C in keratinocytes. Nutr Rev. 2005;63(3): 81–90.
- Kligman LH, Duo CH, Kligman AM. Topical retinoic acid enhances the repair of ultraviolet damaged dermal connective tissue. Connect Tissue Res. 1984;12(2):139–150.
- Olson JA. Serum levels of vitamin A and carotenoids as reflectors of nutritional status. J Natl Cancer Inst. 1984;73(6):1439–1444.
- Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed Proc. 1976;35(6):1332–1338.
- Benaron DA, Cheong WF, Stevenson DK. Tissue optics. Science. 1997;276(5321):2002–2003.
- Pietrzak M, Halicka HD, Wieczorek Z, et al. Attenuation of acridine mutagen ICR-191–DNA interactions and DNA damage by the mutagen interceptor chlorophyllin. Biophys Chem. 2008;135(1–3):69–75.
- Tearney GJ, Brezinski ME, Bouma BE, et al. In vivo endoscopic optical biopsy with optical coherence tomography. Science. 1997;276(5321):2037–2039.
- Jubert C, Mata J, Bench G, et al. Effects of chlorophyll and chlorophyllin on low-dose aflatoxin B(1) pharmacokinetics in human volunteers. Cancer Prev Res (Phila). 2009;2(12):1015–1022.
- Qu J, Ma L, Zhang J, Jockusch S, Washington I. Dietary chlorophyll metabolites catalyze the photoreduction of plasma ubiquinone. Photochem Photobiol. 2013;89(2):310–313.
- Xu C, Zhang J, Mihai DM, Washington I. Light-harvesting chlorophyll pigments enable mammalian mitochondria to capture photonic energy and produce ATP. J Cell Sci. 2014;127(Pt 2):388–399.
- Zmitek K, Pogacnik T, Mervic L, et al. The effect of dietary intake of coenzyme Q10 on skin parameters and condition: results of a randomized, placebo-controlled, double-blind study. Biofactors. 2017; 43(1):132–140.
- Bentinger M, Brismar K, Dallner G. The antioxidant role of coenzyme Q. Mitochondrion. 2007;7 Suppl:S41–S50.
- Littarru GP, Tiano L. Clinical aspects of coenzyme Q10: an update. Nutrition 2010;26(3):250–254.
- Mellors A, Tappel AL. The inhibition of mitochondrial peroxidation by ubiquinone and ubiquinol. J Biol Chem. 1966;241(19):4353–4356.
- Manach C, Scalbert A, Morand C, et al. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–747.
- Scalbert A, Manach C, Morand C, et al. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45(4):287–306.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302(2):71–83.
- Yoon HS, Kim JR, Park GY, et al. Cocoa flavanol supplementation influences skin conditions of photo-aged women: a 24-week double-blind, randomized, controlled trial. J Nutr. 2016;146(1): 46–50.
- Guillou S, Ghabri S, Jannot C, et al. The moisturizing effect of a wheat extract food supplement on women’s skin: a randomized, double-blind placebo-controlled trial. Int J Cosmet Sci. 2011;33(2): 138–143.
- Larmo PS, Yang B, Hurme SA, et al. Effect of a low dose of sea buckthorn berries on circulating concentrations of cholesterol, triacylglycerols, and flavonols in healthy adults. Eur J Nutr. 2009;48(5):277–282.
- Palombo P, Fabrizi G, Ruocco V, et al. Beneficial long-term effects of combined oral/topical antioxidant treatment with the carotenoids lutein and zeaxanthin on human skin: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2007;20(4):199–210.
- Schwartz S, Frank E, Gierhart D, Simpson P, Frumento R. Zeaxanthin-based dietary supplement and topical serum improve hydration and reduce wrinkle count in female subjects. J Cosmet Dermatol. 2016;15(4):e13–e20.
- Yang B, Kalimo KO, Mattila LM, et al. Effects of dietary supplementation with sea buckthorn (Hippophae rhamnoides) seed and pulp oils on atopic dermatitis. J Nutr Biochem. 1999;10(11): 622–630.
- Galland L. Diet and inflammation. Nutr Clin Pract. 2010;25(6):634–640.
- Orengo IF, Black HS, Wolf JE, Jr. Influence of fish oil supplementation on the minimal erythema dose in humans. Arch Dermatol Res. 1992;284(4):219–221.
- Rhodes LE, O’Farrell S, Jackson MJ, Friedmann PS. Dietary fish-oil supplementation in humans reduces UVB-erythemal sensitivity but increases epidermal lipid peroxidation. J Invest Dermatol. 1994;103(2):151–154.
- Rhodes LE, Shahbakhti H, Azurdia RM, et al. Effect of eicosapentaenoic acid, an omega-3 polyunsaturated fatty acid, on UVR-related cancer risk in humans. An assessment of early genotoxic markers. Carcinogenesis. 2003;24(5):919–925.
- Cosgrove MC, Franco OH, Granger SP, et al. Dietary nutrient intakes and skin-aging appearance among middle-aged American women. Am J Clin Nutr. 2007;86(4):1225–1231.
- Boelsma E, Hendriks HF, Roza L. Nutritional skin care: health effects of micronutrients and fatty acids. Am J Clin Nutr. 2001;73(5):853–864.
- Horrobin DF. Essential fatty acid metabolism and its modification in atopic eczema. Am J Clin Nutr. 2000;71(1 Suppl):367S–372S.
- Sies H, Stahl W. Nutritional protection against skin damage from sunlight. Annu Rev Nutr. 2004;24: 173–200.
- Bergouignan A, Momken I, Schoeller DA, et al. Metabolic fate of saturated and monounsaturated dietary fats: the Mediterranean diet revisited from epidemiological evidence to cellular mechanisms. Prog Lipid Res. 2009;48(3–4):128–147.
- Gillingham LG, Harris-Janz S, Jones PJ. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids. 2011;46(3):209–228.
- Latreille J, Kesse-Guyot E, Malvy D, et al. Dietary monounsaturated fatty acids intake and risk of skin photoaging. PLoS One. 2012;7(9):e44490.
- Mataix J, Ochoa JJ, Quiles JL. Olive oil and mitochondrial oxidative stress. Int J Vitam Nutr Res. 2006;76(4):178–183.
- Gliszczynska-Swiglo A, Ciska E, Pawlak-Lemanska K, et al. Changes in the content of health-promoting compounds and antioxidant activity of broccoli after domestic processing. Food Addit Contam. 2006;23(11):1088–1098.
- National Institutes of Health. Vitamin E—Fact Sheet for Health Professionals. Available at: https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/. Accessed June 17, 2019.
- Ghavami A, Coward WA, Bluck LJ. The effect of food preparation on the bioavailability of carotenoids from carrots using intrinsic labelling. Br J Nutr. 2012;107(9):1350–1366.
- Jimenez-Monreal AM, Garcia-Diz L, Martinez-Tome M, et al. Influence of cooking methods on antioxidant activity of vegetables. J Food Sci. 2009;74(3):H97–H103.
- United States Department of Agriculture. Available at https://www.usda.gov. Accessed June 17, 2019.
- Lee S, Choi Y, Jeong HS, et al. Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Sci Biotechnol. 2018;27(2): 333–342.
- Gedi, MA, Briars R, Yuseli F, et al. Component analysis of nutritionally rich chloroplasts: recovery from conventional and unconventional green plant species. J Food Sci Technol. 2017; 54(9) 2746–2757.
- Yuan G, Sun B, Yuan J, Wan, Q. Effects of different cooking methods on health-promoting compounds of broccoli. J Zhejiang Univ Sci B. 2009; 10(8): 580–588

