 J Clin Aesthet Dermatol. 2020;13(5):12–18
J Clin Aesthet Dermatol. 2020;13(5):12–18
by Dharm S. Patel, PhD; Karen A. Veverka, PhD; Jes B. Hansen, PhD; Paul S. Yamauchi, MD; Javier Alonso-Llamazares, MD; and Mark Lebwohl, MD
Drs. Patel and Veverka are with LEO Pharma in Madison, New Jersey. Dr. Hansen is with LEO Pharma in Ballerup, Denmark. Dr. Yamauchi is with the Division of Dermatology at the David Geffen School of Medicine at UCLA in Los Angeles, California. Dr. Alonso-Llamazares is with the Department of Dermatology at the VA Medical Center in Miami, Florida. Dr. Lebwohl is with the Department of Dermatology at the Icahn School of Medicine at Mount Sinai in New York, New York.
FUNDING: p-value communications provided medical writing, editing, and publication assistance and was funded by LEO Pharma.
DISCLOSURES: Drs. Patel, Veverka, and Hansen are employees of LEO Pharma. Dr. Yamauchi serves as a consultant, speaker, advisory board participant, or investigator for AbbVie, Amgen, Celgene, Dermira, Galderma, Janssen-Ortho, LEO Pharma, Lilly, Medimmune, Menlo, Novartis, Ortho, Pfizer Inc., Regeneron, Sandoz, Sanofi, and Sun. Dr. Alonso-Llamazares serves as a speaker for Celgene, Dermira, Ortho Dermatologics, Eli Lilly and Company, and UCB. Dr. Lebwohl is an employee of Mount Sinai and receives research funds from: Abbvie, Amgen, Arcutis, Boehringer Ingelheim, Dermavant, Eli Lilly, Incyte, Janssen Research & Development, LLC, LEO Pharma, Ortho Dermatologics, Pfizer Inc., and UCB. and is a consultant for Aditum Bio, Allergan, Almirall, Arcutis, Inc., Avotres Therapeutics, BirchBioMed Inc., BMD skincare, Boehringer-Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Evelo, Facilitate International Dermatologic Education, Foundation for Research and Education in Dermatology, Inozyme Pharma, Kyowa Kirin, LEO Pharma, Meiji Seika Pharma, Menlo, Mitsubishi, Neuroderm, Pfizer Inc., Promius/Dr. Reddy’s Laboratories, Serono, Theravance, and Verrica.
ABSTRACT: Background. There are a variety of treatment options currently available for plaque psoriasis affecting the scalp, yet scalp psoriasis remains one of the most frustrating and difficult-to-manage forms of the disease.
Objective. We investigated the efficacy of fixed-combination calcipotriene 0.005% plus betamethasone dipropionate 0.064% (Cal/BD) foam for the treatment of scalp psoriasis.
Methods. Additional (including post-hoc) analysis was conducted on data from a Phase II, randomized, double-blind, multicenter study of Cal/BD foam for the treatment of psoriasis vulgaris (NCT01536938). A total of 302 patients, ages 18 years or older, with psoriasis vulgaris of at least mild severity (scalp involvement of at least 10%) were included; 100, 101, and 101 patients were randomized to once-daily Cal/BD foam, Cal foam, or BD foam, respectively. Assessments included a severity score for lesion redness, scaliness, and plaque thickness, modified Psoriasis Area and Severity Index (mPASI) score, proportion of patients with reduction of 50 percent or greater in total sign score (TSS-50), and proportion of patients with at least a 75-percent reduction in mPASI score (mPASI-75).
Results. Patients achieved greater improvements in their scalp psoriasis with Cal/BD foam versus BD or Cal foam alone at Week 4 considering mPASI, mPASI-75, and TSS-50 outcomes. After four weeks of treatment, more patients receiving Cal/BD foam had a severity score for redness, scaliness, and thickness indicating “none” or “mild” versus BD foam or Cal foam alone. Improvements on the scalp appear to be consistent with those on the trunk and limbs.
Conclusion. Scalp lesion severity improved considerably and rapidly with a four-week regimen of Cal/BD foam, suggesting that Cal/BD foam is an effective topical treatment option for scalp psoriasis.
Keywords: Calcipotriene, betamethasone dipropionate, foam, scalp psoriasis, target lesion
The hallmark of scalp psoriasis is the manifestation of sharply demarcated erythematous scaling lesions with silver-white scaly patches and plaques that often extend beyond the circumference of the scalp onto the face.1 As one of the first and more frequent manifestations of psoriasis, these scalp lesions can be present at disease onset and can persist for many years.2–4
Significant physical and psychological burden and stress have been associated with scalp psoriasis. Itching and scaling are the most frequent and distressing symptoms.4 Itching can be intense at times and, due to scratching, can lead to the Koebner phenomenon that contributes to the typical asymmetrical manifestation of scalp lesions.5 Additionally, the visibility of the scales, as well as their shedding as dandruff, can be distressing to patients and contribute to significant psychosocial impairments in everyday life, including during personal interactions and work-related activities.5,6 Potential hair loss accompanying scalp psoriasis can add to the already substantial burden of the disease.7–10
Various treatment options that are effective for plaque psoriasis can be used on the scalp, but scalp psoriasis remains one of the most frustrating and difficult-to-manage forms of the disease; this is partly due to the difficulty in reaching the scalp surface when applying topical treatments through the hair.11–13 Topical treatments involving corticosteroids and vitamin D3 analogs are the mainstay of treatment for most patients with plaque psoriasis. Combined formulations are often the preferred option based on their enhanced efficacy and minimized toxicity.11,13
Several clinical studies have demonstrated that the combination of calcipotriene 0.005% (Cal) and betamethasone dipropionate 0.064% (BD) (Cal/BD) shows superior efficacy and a favorable safety profile compared with using either Cal or BD alone in treating scalp psoriasis.14–17 The foam formulation of Cal/BD, in particular, has demonstrated a favorable efficacy and safety profile in the treatment of scalp psoriasis in a Phase II randomized study.18 We conducted additional analyses (including post-hoc) of the Phase II study to expand our understanding of the impact of Cal/BD foam formulation in the treatment of scalp psoriasis, with a focus on the improvement in lesion quality.
Methods
Study design, objectives, and patient population. The original study was a Phase II, randomized, double-blind, multicenter study conducted in the United States between May 2012 and October 2012 (NCT01536938). The details of the study methodology, patient selection, end points, and prospective statistical analyses have been previously described.18
Eligible patients, ages 18 years of age or older, with psoriasis vulgaris of at least mild severity of both the body and scalp for six months or longer were randomized 1:1:1 to once-daily fixed-combination Cal/BD foam, Cal foam, or BD foam for four weeks, followed by a safety follow-up visit two weeks later as needed. The psoriasis involved at least 10 percent of the total scalp area in addition to other body regions (total involvement less than or equal to 30% of the body surface area). The primary efficacy end point in the original study was the achievement of treatment success for body psoriasis at Week 4 according to the Physician’s Global Assessment (PGA; also referred to as the Investigator’s Global Assessment) of disease severity; treatment success was defined as a rating of “clear” or “almost clear” in patients with moderate to severe disease at baseline and of “clear” for those with mild disease at baseline.18 Other assessments included the modified Psoriasis Area and Severity Index (mPASI) score, the percentage change in mPASI score from baseline, and the percentage of patients achieving at least a 75-percent reduction in mPASI (mPASI-75) score at Weeks 1, 2, and 4 of treatment for the scalp, trunk, and limbs.
Additional analyses. The primary objective of this post-hoc analysis was to investigate the efficacy of Cal/BD foam for the treatment of scalp psoriasis in terms of lesion severity as measured by each of the parameters for the mPASI score—redness, scaliness, and plaque thickness—at Weeks 1 and 4. The mPASI parameters were measured on a five-point scale, where 0= none, 1=mild, 2=moderate, 3=severe, and 4=very severe. Response to treatment was assessed by 1) the proportion of patients achieving ratings of “clear” or “almost clear” for lesion redness, scaliness, and thickness; 2) the proportion of patients achieving a reduction of 50 percent or greater in the total sign score (TSS-50), in which TSS is the sum of the scores for the three components of redness, scaliness, and thickness; 3) mPASI score; and 4) the proportion of patients achieving mPASI-75 at Weeks 1, 2, and 4.
Statistical analyses. Binary outcomes for treatment response, the percentage of patients reaching mPASI-75, the percentage of patients achieving “clear” or “almost clear” for each parameter (i.e., redness, scaliness, and thickness), and the percentage of patients achieving TSS-50 were compared between treatment groups using the Cochran-Mantel-Haenszel method, adjusted for pooled centers. Comparisons of mPASI scores for the scalp were conducted using analysis of covariance, including the pooled center, treatment, and baseline mPASI score in the model. Scatterplots of baseline values and postbaseline percentage changes in mPASI score for the scalp versus for the trunk and limbs were plotted and Spearman correlation coefficients were calculated. Missing data were replaced using the last observation carried forward (LOCF) method, unless otherwise stated.
Results
Patients. A total of 302 patients were randomized in the study (Cal/BD foam, n=100; Cal foam, n=101; BD foam, n=101) and the majority of these patients (93%) completed the study. Patient demographics and baseline characteristics were similar between treatment groups and have been previously reported (Table 1).18 Briefly, the mean age across the treatment arms ranged between 47.4 and 50.7 years; most patients were male (53%–60%), white (82%–93%), and had moderate to severe psoriasis of the scalp (77%–82%), with a mean disease duration of 14.6 to 18.4 years. Across the treatment arms, the majority of patients (~83%) showed psoriasis involvement of 10 percent to 49 percent on the scalp, with the mean mPASI score ranging between 0.84 and 0.90 points.
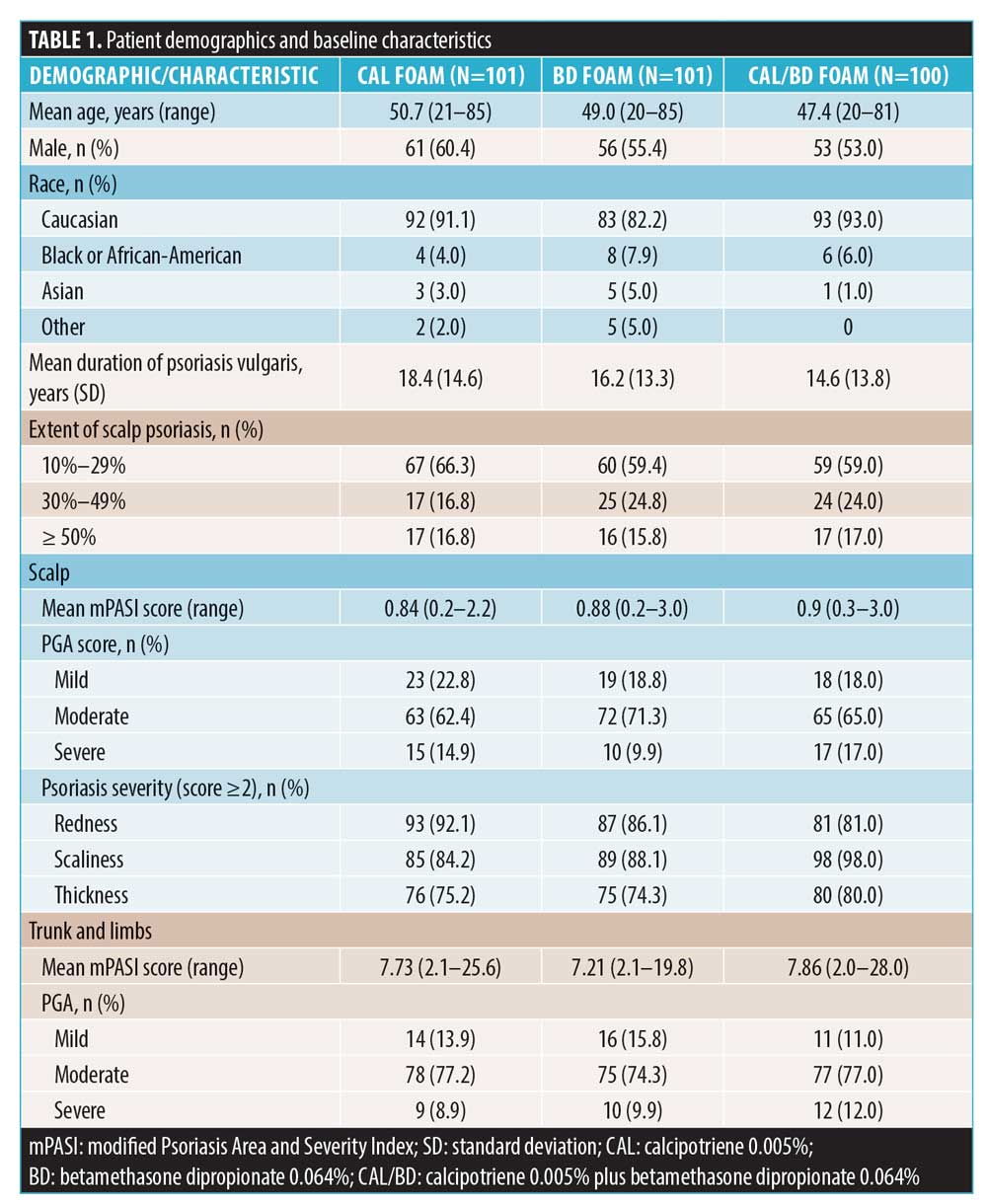
Efficacy and safety analyses. The mean mPASI score for the scalp improved from baseline in all groups, with Cal/BD foam patients achieving a significantly greater improvement at Week 4 compared to the Cal foam patients (0.18 vs. 0.37; p<0.001), but not to patients treated with BD foam (0.18 vs. 0.26; p=0.058) (Figure 1A). Correspondingly, the mean percentage reduction in mPASI score at the scalp for Cal/BD foam patients was greater than that for Cal or BD foam patients at Week 4 (–79.98% vs. –71.19% vs. –57.79%) (Figure 1B). Additionally, the proportion of patients achieving mPASI-75 with Cal/BD foam for the scalp was significantly greater than that seen with Cal foam (73.0% vs. 50.5%; p=0.002), and was numerically but not statistically higher than that seen with BD foam (73.0% vs. 65.3%; p=0.25) at Week 4 (Figure 1C).
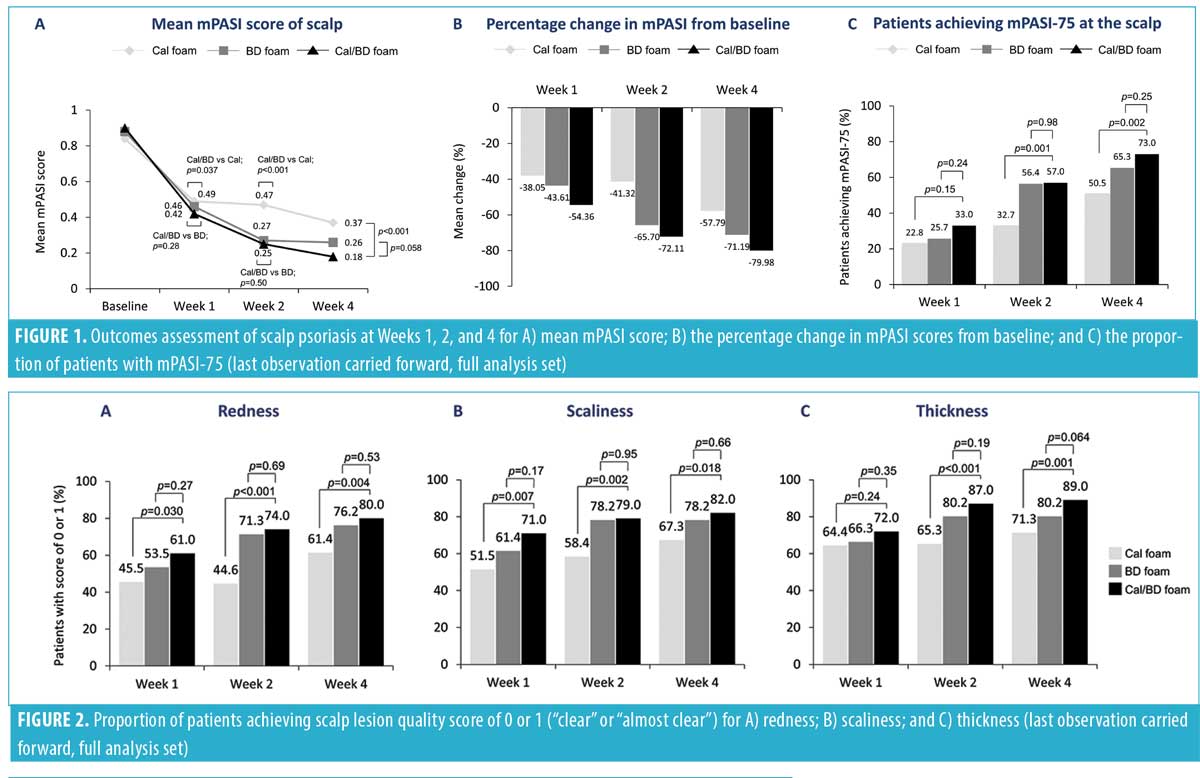
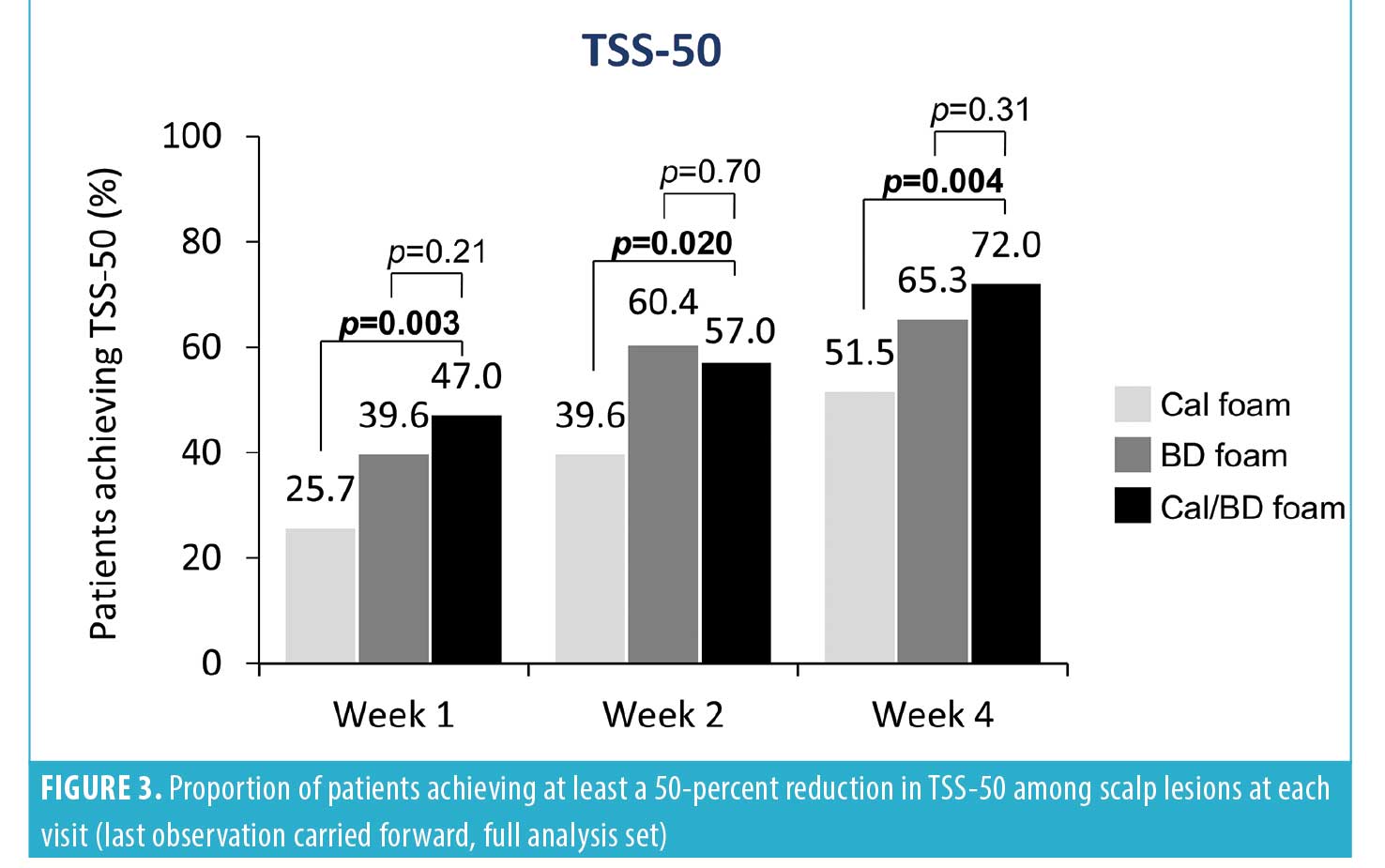
Post-hoc analyses were performed for each of the mPASI parameters (i.e., redness, scaliness, and thickness) for the scalp lesions at Weeks 1, 2, and 4. A greater proportion of patients achieved considerable improvement (a score of 0 or 1) in the severity of scalp lesions with Cal/BD foam vs. BD or Cal foam at Week 4 for redness (80.0% vs. 76.2% vs. 61.4%, respectively), scaliness (82.0% vs. 78.2% vs. 67.3%), and thickness (89.0% vs. 80.2% vs. 71.3%). Statistically significant differences were observed for Cal/BD versus Cal foam (Figure 2). Notably, the majority (61%–72%) of patients reported improvements in scalp lesion quality with Cal/BD foam as early as one week after starting treatment (Figure 2). Correspondingly, more patients using Cal/BD foam also achieved at least a 50-percent improvement in the severity score (TSS-50) at Week 4 (72.0%), compared to 65.3 percent of patients using BD foam and 51.5 percent using Cal foam; a statistically significant difference was again observed for Cal/BD foam versus Cal foam (Figure 3).
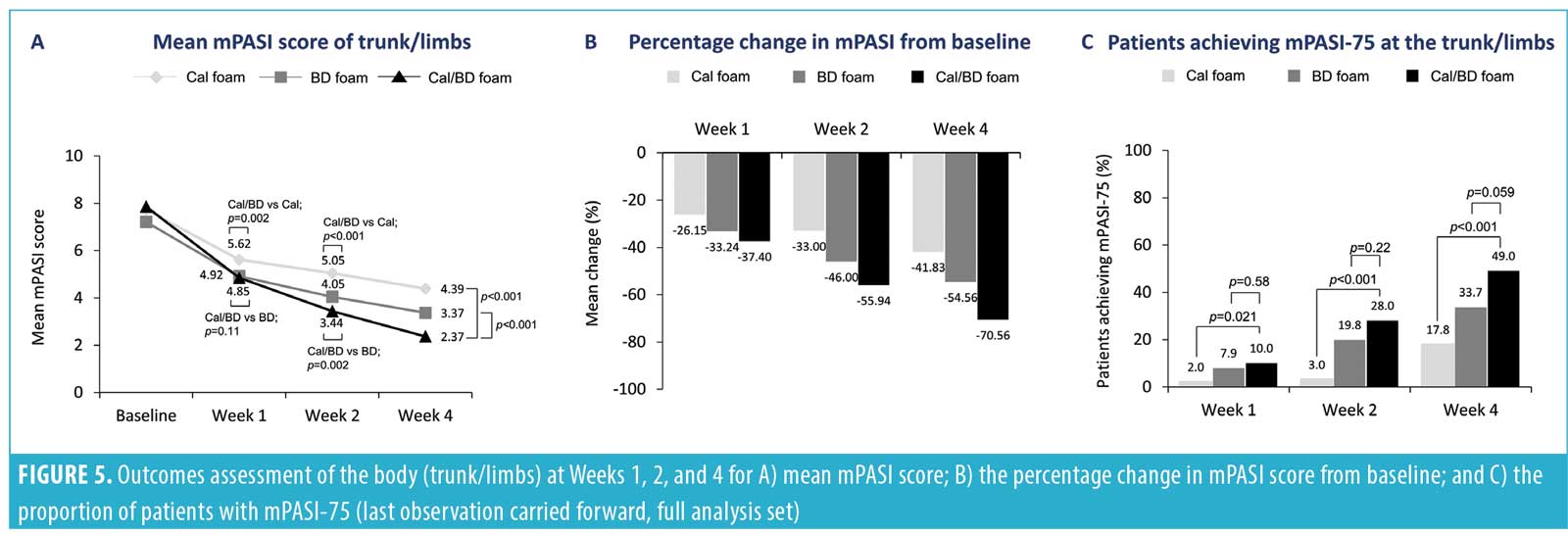
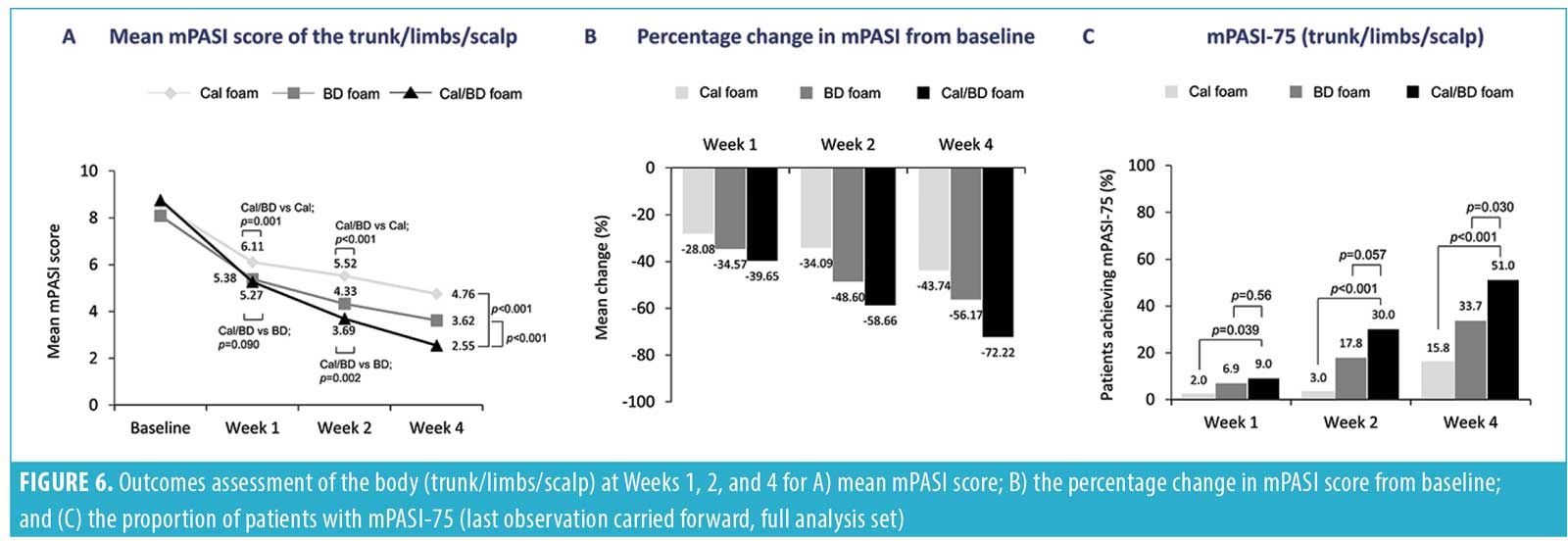
 The improvements in mPASI scores at the scalp with a four-week regimen of Cal/BD foam also appeared to trend consistently with the improvements in psoriasis severity that have been noted for the trunk plus limbs18 and for the entire body (trunk plus limbs plus scalp) (Figures 5 and 6). Indeed, the Spearman correlation for the post-baseline percentage changes in mPASI score between the scalp and the trunk and limbs ranged from weak to moderate for Cal/BD foam at Weeks 1, 2, and 4; all correlations were statistically significant (p<0.0001). The scatterplots for the correlations are shown in Figure 4.
The improvements in mPASI scores at the scalp with a four-week regimen of Cal/BD foam also appeared to trend consistently with the improvements in psoriasis severity that have been noted for the trunk plus limbs18 and for the entire body (trunk plus limbs plus scalp) (Figures 5 and 6). Indeed, the Spearman correlation for the post-baseline percentage changes in mPASI score between the scalp and the trunk and limbs ranged from weak to moderate for Cal/BD foam at Weeks 1, 2, and 4; all correlations were statistically significant (p<0.0001). The scatterplots for the correlations are shown in Figure 4.

The safety and tolerability of Cal/BD treatment on the scalp appeared to be favorable. Only two cases of cutaneous adverse reactions—application site pain and alopecia—were reported with Cal/BD foam application; the relationship to the scalp was unknown.
Discussion
The additional analyses of a Phase II study of Cal/BD foam showed that a majority of patients achieved considerable improvements in their scalp psoriasis after four weeks of treatment. There was a rapid onset of efficacy with once-daily Cal/BD foam as early as after one week of treatment in many patients, and improvements at the scalp also correlated with those observed at the trunk and limbs.
Individual PASI parameters and the TSS, a common assessment tool for scalp psoriasis, were used to evaluate the severity of scalp psoriasis in terms of redness, amount of scaling, and thickness of the lesions.19 The majority of patients achieved considerable improvements in all three qualities of the scalp lesions after four weeks of treatment with Cal/BD foam and many did so by the one-week assessment. In almost all patients (80%–90%), the redness, scaliness, and thickness of lesions had either resolved or were reduced to a mild severity with treatment from a baseline rating of moderate, severe, or very severe. These improvements in lesion quality also appeared to trend consistently with other global efficacy assessments, such as mPASI score.
Although global assessments are commonly used in clinical studies and provide all-encompassing scores for disease status, observations directed to lesion quality might provide a better indication of treatment outcomes that are most relevant to patients. Redness and scaling in particular are frequently present, with scaling and itching being among the most troubling symptoms.4 The improvements in scalp lesion quality shown in this study highlight benefits from Cal/BD foam that can be directly perceived and appreciated by patients. Interestingly, the observed consistency in scalp improvements, together with those observed in other parts of the body, occurred regardless of the type of outcome assessment used (e.g., treatment success, mPASI, mPASI-75) and were consistent with findings reported in the original study.18 The benefits of Cal/BD foam in alleviating body psoriasis have been well established, and the results of this study further suggest that patients might expect similar benefits when using it to treat scalp psoriasis.18,20–24
Impact of betamethasone and calcipotriene in the combination formulation. In this study, the foam combination of calcipotriene and betamethasone produced greater benefits than either betamethasone or calcipotriene monotherapy in the treatment of scalp psoriasis, a finding that is consistent with the previously published results of the original study as well as of studies using gel or ointment formulations of the combination, including the original study.18,25–27 In the original study, 53 percent of patients using Cal/BD foam achieved treatment success of scalp psoriasis, defined as PGA score of “clear” or “almost clear” from baseline, relative to smaller percentages for BD foam (47.5%; p=0.45) and Cal foam (35.6%; p=0.021). Improvements in scalp psoriasis in the original study were also observed as early as Week 1.18 The efficacy of combination therapy has been substantiated by a Cochrane systematic review of 59 randomized clinical trials involving more than 11,000 participants up to 2015, which showed that a corticosteroid and its combination formulation with a vitamin D analog was more effective than a vitamin D analog alone in clearing lesions and eliciting a treatment response as measured by both Investigator’s Global Assessment and PGA scores.28 Betamethasone in itself was also associated with a significantly greater improvement in psoriasis on the scalp and face than calcipotriene in a previous study of 474 patients treated twice daily for four weeks.29
These observations are, in part, attributable to the stronger suppression of proinflammatory cytokines, such as interleukin (IL)-17A, IL-22, and tumor necrosis factor alpha (TNF-alpha), by a corticosteroid alone and by the combination formulation of a steroid combined with a vitamin D analog versus a vitamin D analog alone.30 Nonetheless, the inhibition of cytokine release (e.g., IL-23, TNF-alpha) has been found to be driven mostly by calcipotriene, which suggests that both components can contribute to the synergistic effect of the combination formulation, thus highlighting the importance of the combination formulation over monotherapy.30
Notwithstanding the efficacy profile, the combination formulation of Cal/BD is often the preferred choice of therapy because it avoids harmful side effects, such as skin atrophy associated with superpotent steroids and skin irritation associated with Cal monotherapy.31,32 A meta-analysis of 10 randomized clinical studies of Cal/BD for the treatment of scalp psoriasis showed significantly lower rates of adverse events, including skin-related events, and lower rates of study discontinuation due to adverse events with Cal/BD compared to Cal or BD alone.25 In the original study on which our post-hoc analysis was based, all three treatment options were reported as safe and well tolerated, with a low overall incidence of adverse events and with most adverse events being mild to moderate in severity.18 Only two skin-related adverse events—one case of alopecia and one case of application site pain—were reported for Cal/BD foam; however, it is unclear as to whether these adverse events were related to the scalp.
Foam as a delivery vehicle. A key obstacle to effectively treating the scalp is that hair can render the scalp relatively inaccessible to topical dermatologic therapies, which reduces the convenience and ease of use of topical therapies and ultimately impacts the effectiveness of treatment. A favorable vehicle for delivering active agents for treating scalp psoriasis is one that is not messy or unpleasant when applying and is easy to spread onto the skin surface of the scalp.4,12 Among the different options, foam is often considered a superior vehicle and has been preferred by patients to gels and ointments in terms of convenience, ease of application, and overall impact on quality of life.33–35 Thus, the perception of foam as a favorable topical vehicle might enable patients to remain more adherent to the treatment, thereby obtaining its full benefit.36
Limitations. One limitation of a post-hoc analysis is that the original study was not powered for comparisons of the scalp-specific assessments used in this study, and therefore the different statistical comparisons should be interpreted with caution. Nonetheless, the current study is an important initiative to help bridge the gap in understanding how best to manage scalp psoriasis with corticosteroid and vitamin D analog topical therapies. In addition, the results of our analysis are consistent with the initial assessment of Cal/BD effectiveness for scalp psoriasis in the original study as well as of other formulations in previous investigations.18,37,38 Larger studies are needed to validate the current results.
Conclusion
Considerable improvements in scalp lesion severity were achieved with a four-week regimen of once-daily Cal/BD foam. Rapid onset of efficacy was observed as early as Week 1. Overall, the findings of this study suggest that Cal/BD foam is an effective topical treatment option for scalp psoriasis.
References
- van de Kerkhof PC, Franssen ME. Psoriasis of the scalp. Diagnosis and management. Am J Clin Dermatol. 2001;2(3):159–165.
- Farber EM, Nall L. Natural history and treatment of scalp psoriasis. Cutis. 1992;49(6):396–400.
- Kaur I, Handa S, Kumar B. Natural history of psoriasis: a study from the Indian subcontinent.
J Dermatol. 1997;24(4):230–234. - van de Kerkhof PC, de Hoop D, de Korte J, et al. Scalp psoriasis, clinical presentations and therapeutic management. Dermatology. 1998;197(4):326–334.
- Kragballe K, Menter A, Lebwohl M, et al. Long-term management of scalp psoriasis: perspectives from the International Psoriasis Council. J Dermatolog Treat. 2013;24(3):188–192.
- Blakely K, Gooderham M. Management of scalp psoriasis: current perspectives. Psoriasis (Auckl). 2016;6:33–40.
- van de Kerkhof PC, Chang A. Scarring alopecia and psoriasis. Br J Dermatol. 1992;126(5):524–525.
- Criado PR, Valente NY, Michalany NS, et al. An unusual association between scalp psoriasis and ophiasic alopecia areata: the Renbok phenomenon. Clin Exp Dermatol. 2007;32(3):320–321.
- Cockayne SE, Messenger AG. Familial scarring alopecia associated with scalp psoriasis. Br J Dermatol. 2001;144(2):425–427.
- Runne U, Kroneisen-Wiersma P. Psoriatic alopecia: acute and chronic hair loss in 47 patients with scalp psoriasis. Dermatology. 1992;185(2):82–87.
- Ortonne J, Chimenti S, Luger T, et al. Scalp psoriasis: European consensus on grading and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:1435–1444.
- Papp K, Berth-Jones J, Kragballe K, et al. Scalp psoriasis: a review of current topical treatment options. J Eur Acad Dermatol Venereol. 2007;21(9):1151–1160.
- Chan CS, Van Voorhees AS, Lebwohl MG, et al. Treatment of severe scalp psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2009;60(6): 962–971.
- McCormack PL. Spotlight on calcipotriene/betamethasone dipropionate in psoriasis vulgaris of the trunk, limbs, and scalp. Am J Clin Dermatol. 2011;12(6):421–424.
- van de Kerkhof PC, Hoffmann V, Anstey A, et al. A new scalp formulation of calcipotriol plus betamethasone dipropionate compared with each of its active ingredients in the same vehicle for the treatment of scalp psoriasis: a randomized, double-blind, controlled trial. Br J Dermatol. 2009;160(1):170–176.
- Luger TA, Cambazard F, Larsen FG, et al. A study of the safety and efficacy of calcipotriol and betamethasone dipropionate scalp formulation in the long-term management of scalp psoriasis. Dermatology. 2008;217(4):321–328.
- Jemec GB, Ganslandt C, Ortonne JP, et al.
A new scalp formulation of calcipotriene plus betamethasone compared with its active ingredients and the vehicle in the treatment of scalp psoriasis: a randomized, double-blind, controlled trial. J Am Acad Dermatol. 2008;59(3):455–463. - Lebwohl M, Tyring S, Bukhalo M, et al. Fixed combination aerosol foam calcipotriene 0.005% (Cal) plus betamethasone dipropionate 0.064% (BD) is more efficacious than Cal or BD aerosol foam alone for psoriasis vulgaris: a randomized, double-blind, multicenter, three-arm, phase 2 study. J Clin Aesthet Dermatol. 2016;9(2):34–41.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis?: quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130(4):933–943.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15(8):951–957.
- Menter A, Gold LS, Koo J, et al. Fixed-combination calcipotriene plus betamethasone dipropionate aerosol foam is well tolerated in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. Skinmed. 2017;15(2):119–124.
- Paul C, Stein Gold L, Cambazard F, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs. gel in patients with psoriasis vulgaris: randomized, controlled PSO-ABLE study. J Eur Acad Dermatol Venereol. 2017;31(1):119–126.
- Koo J, Tyring S, Werschler WP, et al. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris—a randomized phase II study. J Dermatolog Treat. 2016;27(2):120–127.
- Leonardi C, Bagel J, Yamauchi P, et al. Efficacy and safety of calcipotriene plus betamethasone dipropionate aerosol foam in patients with psoriasis vulgaris–a randomized phase III study (PSO-FAST). J Drugs Dermatol. 2015;14(12): 1468–1477.
- Bottomley JM, Taylor RS, Ryttov J. The effectiveness of two-compound formulation calcipotriol and betamethasone dipropionate gel in the treatment of moderately severe scalp psoriasis: a systematic review of direct and indirect evidence. Curr Med Res Opin. 2011;27(1):251–268.
- Buckley C, Hoffmann V, Shapiro J, et al. Calcipotriol plus betamethasone dipropionate scalp formulation is effective and well tolerated in the treatment of scalp psoriasis: a phase II study. Dermatology. 2008;217:107–113.
- Ma L, Yang Q, Yang H, et al. Calcipotriol plus betamethasone dipropionate gel compared with calcipotriol scalp solution in the treatment of scalp psoriasis: a randomized, controlled trial investigating efficacy and safety in a Chinese population. Int J Dermatol. 2016;55(1):106–113.
- Schlager JG, Rosumeck S, Werner RN, et al. Topical treatments for scalp psoriasis: summary of a Cochrane Systematic Review. Br J Dermatol. 2017;176(3):604–614.
- Klaber MR, Hutchinson PE, Pedvis-Leftick A, et al. Comparative effects of calcipotriol solution (50 micrograms/ml) and betamethasone 17-valerate solution (1 mg/ml) in the treatment of scalp psoriasis. Br J Dermatol. 1994;131(5):678–683.
- Lovato P, Norsgaard H, Tokura Y, Røpke MA, et al. Calcipotriol and betamethasone dipropionate exert additive inhibitory effects on the cytokine expression of inflammatory dendritic cell-Th17 cell axis in psoriasis. J Dermatol Sci. 2016;81(3): 153–164.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–659.
- Segaert S, Ropke M. The biological rationale for use of vitamin d analogs in combination with corticosteroids for the topical treatment of plaque psoriasis. J Drugs Dermatol. 2013;12(8):e129–137.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70(6):327–332.
- Feldman SR, Housman TS. Patients’ vehicle preference for corticosteroid treatments of scalp psoriasis. Am J Clin Dermatol. 2003;4(4):221–224.
- Bergstrom KG, Arambula K, Kimball AB. Medication formulation affects quality of life: a randomized single-blind study of clobetasol propionate foam 0.05% compared with a combined program of clobetasol cream 0.05% and solution 0.05% for the treatment of psoriasis. Cutis. 2003;72(5):407–411.
- Choi JW, Kim BR, Youn SW. Adherence to topical therapies for the treatment of psoriasis: surveys of physicians and patients. Ann Dermatol. 2017;29(5):559–564.
- Feldman SR, Ravis SM, Fleischer AB Jr., et al. Betamethasone valerate in foam vehicle is effective with both daily and twice a day dosing: a single-blind, open-label study in the treatment of scalp psoriasis. J Cutan Med Surg. 2001;5(5): 386–389.
- Mazzotta A1, Esposito M, Carboni I, et al. Clobetasol propionate foam 0.05% as a novel topical formulation for plaque-type and scalp psoriasis. J Dermatolog Treat. 2007;18(2):84–87.

