 J Clin Aesthet Dermatol. 2021;14(5):E61–E69.
J Clin Aesthet Dermatol. 2021;14(5):E61–E69.
by Gillian Murray, MPharm, PG Dip Clin Pharm, INP; Cormac Convery, MB ChB, MSc, MASLMS; Lee Walker, BDS, MFDS, RCPSG, MJDF, RCS, ENG; and Emma Davies, RN INP
Dr. Murray is with Clinical Academic Kings College in London, England. Dr. Convery is with The Ever Clinic in Glasgow, Scotland. Dr. Walker is with B City Clinic in Liverpool, England. Ms. Davies is Clinical Director of Save Face in Cardiff, United Kingdom. All authors are founding board members of the Complications in Medical Aesthetics Collaborative (CMAC).
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Vascular occlusions can occur with injection of dermal fillers causing devastating outcomes for the patient. The occurrence, and subsequent management, of these negative outcomes is a source of significant stress to the aesthetic clinician. Complications management is an essential component of clinical practice and professionals must develop competence and confidence in the identification and effective treatment of a vascular occlusion. The relatively rare occurrence of a vascular occlusion mandates that learning must be largely through the study of theory in addition to the sharing of learning experiences within a collaborative clinical community. The delivery of optimal care begins with an understanding of the underlying pathophysiology and the ability to assess and elicit clinical signs. Establishing a clinical diagnosis, targeted therapy can commence in a timely fashion. This paper provides guidance on how to identify and manage a vascular occlusion caused by cross-linked hyaluronic acid. It provides a detailed description of the pathological process of tissue ischemia, and introduces identifiable stages which will help to determine the extent of ischemia and the time frame since ischemic onset. The stages are particularly important as they highlight when wound support may be needed.
Keywords: Vascular occlusion, dermal filler, cross-linked hyaluronic acid, filler, complication, necrosis, hyaluronidase, hyaluronic acid, non-surgical
A vascular occlusion is a potentially severe adverse outcome that can occur when hyaluronic acid filler is accidentally injected into a blood vessel. The number of dermal filler treatments performed globally is rising; according to a study by Belezney et al,1 the number has grown by 300 percent from 2000 to 2017. As the number and complexity of procedures increases, the incidence of vascular occlusions will likely also increase.1
The absence of regulation in aesthetic practice in the United Kingdom has resulted in rising levels of inexperience and incompetence in regard to diagnosing and managing adverse events secondary to dermal filler treatments. As a result of this lack of clear management pathways, the Complications in Medical Aesthetics Collaborative (CMAC) is seeing an increase in patients with vascular events who present for treatment at a later stage, requiring more complex treatment plans to achieve optimal recovery.
Hyaluronic acid fillers can be implanted through all tissue planes, bringing an associated risk of inadvertent intravascular injection. If a vascular occlusion is not promptly diagnosed and managed appropriately, tissue necrosis can ensue. Outcomes are all the more catastrophic when involving anastomotic connections between the external and internal carotid arteries; in these circumstances, blindness and stroke are both possible. According to animal studies, the retina can tolerate 97 minutes of hypoxia before damage becomes irreversible.2 However, more recent research has demonstrated that a vascular occlusion can cause a retinal infarction in 12 to 15 minutes.3
Clinicians must work to minimize risks by adopting a safer injection technique and having a thorough knowledge of three-dimensional facial anatomy. They must be able to recognize the clinical presentation of ischemia, having clear protocols and referral pathways in place.
This guideline has been developed to support clinicians in the minimization of risk during treatment. In the event that a vascular occlusion does occur following treatment, the guideline will assist in the assessment, diagnosis, and management of the situation, with the ultimate aim being the prevention of tissue necrosis.
Causes of Vascular Occlusion
In addition to direct intravascular occlusion by hyaluronic acid fillers, some authors hypothesize that compression or vascular spasm can give rise to ischemic changes in the tissue. Compression has been difficult to replicate in animal models.4
Ischemia and its effects on tissue can give rise to vasospasm. When the tissue is ischemic, the vasospasm occurs because of desensitization to nitrous oxide. Combined with compression due to extravascular edema, changes in the surrounding tissue can be expected. Further, hyaluronic acid filler, when injected intravascularly, can act as a noxious stimulus, producing inflammation and further intense vasospasm.5 These secondary vascular changes will worsen the ischemia caused by the direct intravascular occlusion. Ultimately, there is a need for more research detailing precise vascular models leading to ischemic changes after injection of hyaluronic acid filler pertaining to different facial zones (i.e., vascular territories).
Ischemic changes typically occur instantly or within a few hours.3 Accompanying symptoms and signs can be variable, and it is of the utmost importance that the clinician has a high index of suspicion when seeking their presence. On rare occasions, the onset of ischemia and the accompanying symptoms and signs may occur a few days later; it is not fully understood why this might occur. However, a number of possible explanations have been hypothesized:
- It is possible that an embolic event in a distal (to the site of injection), narrower arteriole can cause delayed ischemic changes.
- The hygroscopic nature of the hyaluronic acid leads to an increase in bolus size, causing a more complete occlusion or compression in predisposed areas outside the vessel.
- The initial bolus might not be large enough to fully occlude the vessel. However, the vessel subsequently becomes occluded through platelet aggregation.
Recent work by Taylor et al5 described patterns of ischemia in relation to the ophthalmic artery and it’s corresponding angiosomal territory. They found that direct venous injection, or movement of the hyaluronic acid emboli via an arteriovenous shunt, could result in a delayed mixed pattern of ischemia and necrosis. They found that the combination of large caliber, avalvular veins that connect to each orbit permitting flow in each direction render the ophthalmic artery vulnerable to inadvertent injection with hyaluronic acid.5
Regardless of the etiology of the occlusion or when the ischemic changes present, the clinician should be driven by the clinical signs and first treat to recover perfusion and then to support healing as required.
Mitigating the Risks of Vascular Occlusion
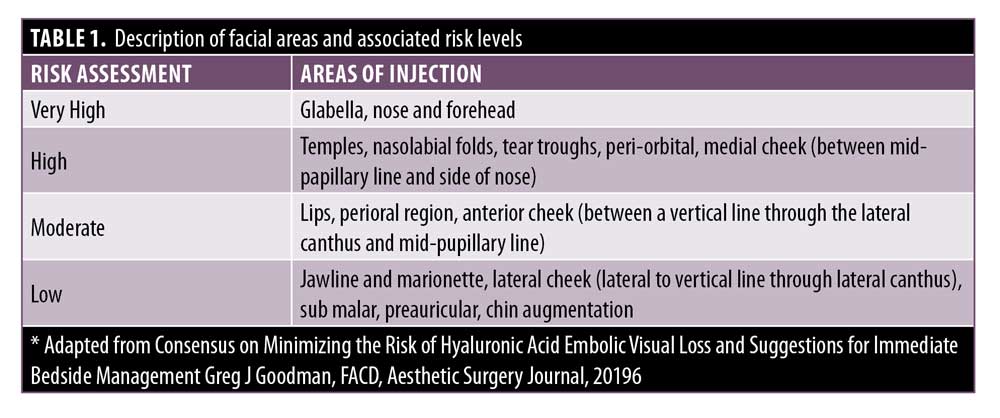
Anatomy. The importance of understanding and respecting anatomy should never be underestimated. Target areas for injection of soft tissue fillers should be graded according to risk and never described as areas that are safe. “Safe” implies no risk, and this can be misleading and dangerous. There is always a risk when the needle breaches the skin envelope. The clinician should always consider the level of risk associated with a given area when planning a treatment (Table 1). To assist in visualization of these anatomical risk zones, Figure 1 identifies the area of the face separating them into low, moderate, high, and very high.

An appreciation of both the depth and distribution of structures will lead to a lower risk and more predictable way to inject soft-tissue fillers. Injection anatomy is anatomy pertinent to where the tip of the needle is located within the tissue layers. This is vastly different to surgical and radiographical anatomy. Standard anatomical text normally highlights the distribution of the structures (i.e., vasculature and nerves). This is a two-dimensional learning approach, while the face is a three-dimensional structure. Therefore, teaching and learning of anatomical structures requires the understanding of depth in relation to the tissue planes.
Cannula versus needle. Vascular complications can occur when using either a needle or a cannula. Unfortunately, in many of the cases relating to visual disturbance, the technique is not stated. In a review of cases of visual disturbance by Belezney et al,1 the authors reported only 33.3 percent of these cases included details regarding usage of either needle or cannula. A needle was used in 10 cases and cannula in six. The cannula gauges ranged from 2g to 23g. A 27-g cannula was found to penetrate an artery, like a needle, from the same applied force. When using a needle, it is possible to pass through a vessel and for subsequent retrograde flow of the filler to pass back through the needle track and into the vessel.1
More recently published data seems to suggest that the safety associated with use of a cannula might be overestimated. Zhou et al7 reported 28 cases of severe hyaluronic acid embolism, nine cases of blindness, one case of blindness with stroke, and 18 cases of large area necrosis; it was found that 25 of these 28 patients were injected using cannula (22–27g), instead of needles. They do state that cannulas smaller than 25g when injecting filler should be avoided, and this is the consensus among the CMAC board. Regardless of the technique, all safety measures and checks should apply.
Aspiration. The subject of aspiration is widely discussed. Clinicians should not rely on aspiration as their sole safety check. Published evidence from Casabona9 found the reliability of aspiration to be 53 percent, while Van Loghem10 recorded reliability rates between 33 and 63 percent. Accuracy of aspiration is dependent upon needle diameter, time applying negative pressure to the plunger, whether the needle is primed, and needle length. Torbeck et al10 suggested that the rheology of the filler is the main factor in gaining a true positive aspirate. A systematic review published by Kapoor et al11 reported a pooled data analysis as a result of their systematic review. They found that there was an association between filler elastic modulus (G’), cohesivity (by drop weight), and cross-section of needle lumen. There was not sufficient evidence to confirm an association between the product in general, needle, and pullback volume. This means it is not possible to establish the ideal aspiration procedure for all product variations to achieve an accurate aspiration. Consequently, a negative aspirate does not mean the injection will be safe, and a vascular occlusion cannot be ruled out if there are signs of ischemic changes in the tissue.11 It should also be noted that certain techniques, such as linear threading and fanning, do not lend themselves to aspiration as a tool to identify vascular cannulation.
Products. Product knowledge is essential. Hyaluronic acid fillers have different rheological and physicochemical properties, and this can impact how certain products dissolve upon exposure to hyaluronidase.12 There is very limited research detailing how different hyaluronic acid filler brands dissolve in comparison to each other, and there is very little known about the respective chemistry of many hyaluronic acid fillers available globally. There are anecdotal findings that some products resist enzymatic breakdown more than others, but more research is needed in this area.
Communication. It is important to open a dialogue of communication with each patient. Pain is subjective and anything beyond what is expected should be communicated immediately. Some discomfort is usual. However, severe pain distal to the injection site or alterations in sensation is not normal.
Good practice. It is possible to reduce both the likelihood and extent of a vascular occlusion by improving the knowledge of injection anatomy and ensuring a safer technique is used, as outlined in Table 2.4
Assessing a Suspected Vascular Occlusion
Vascular occlusions might be immediately apparent or presentation might be delayed, sometimes presenting hours or even days after the treatment.4
It is important that a clinician can effectively assess capillary refill time, as this will allow prompt identification of tissue ischemia and assist in determining the total area of ischemia.
Assessing capillary refill time. It is vital to assess the capillary refill time (CRT) along the distribution of the artery immediately after injection. It is advisable to allow 30 to 45 minutes to assess a patient after injection of high-risk areas, such as the nose, glabella, or forehead. The CRT—the amount of time needed for the blood in a peripheral area of the body to return after compression—must be checked on both sides of the corresponding region of the face and is considered normal if it is less than two seconds. A brisk CRT can indicate venous insufficiency.7
Pain. Some discomfort during the procedure is normal. However, if local anesthetic has been used either in combination with the hyaluronic acid or injected separately, the pain can be masked until the anesthesia wears off. Sudden escalating pain during treatment either at the site of the injection or in a distant site is not normal. The injection should be stopped immediately and the tissue assessed. If a patient complains of worsening post-procedure pain, they must be reviewed and assessed.4,14 It’s important to note that, in event of a vascular occlusion, pain is not always present during the early stages.
Skin color. Skin color is an important marker of ischemic changes. The skin signs can be explained in Stages 1 to 5 (Figure 2A–2E) to assist in predicting whether a good recovery can be expected or if there is likely to be tissue breakdown and a wound that will require management. Once the blood supply has been interrupted or restricted, the tissue can appear pale or dusky. The pallor, or blanching, may be fleeting but will be replaced by a reticulated purple pattern as the deoxygenated blood in the tissue builds up.4 It is important to assess the entire face, especially along the tract of the artery and communicating vessels, as the filler can travel into more distant branches of the vasculature. Any pallor or dusky appearance and/or progression into a reticulated pattern requires immediate treatment to the entire area.
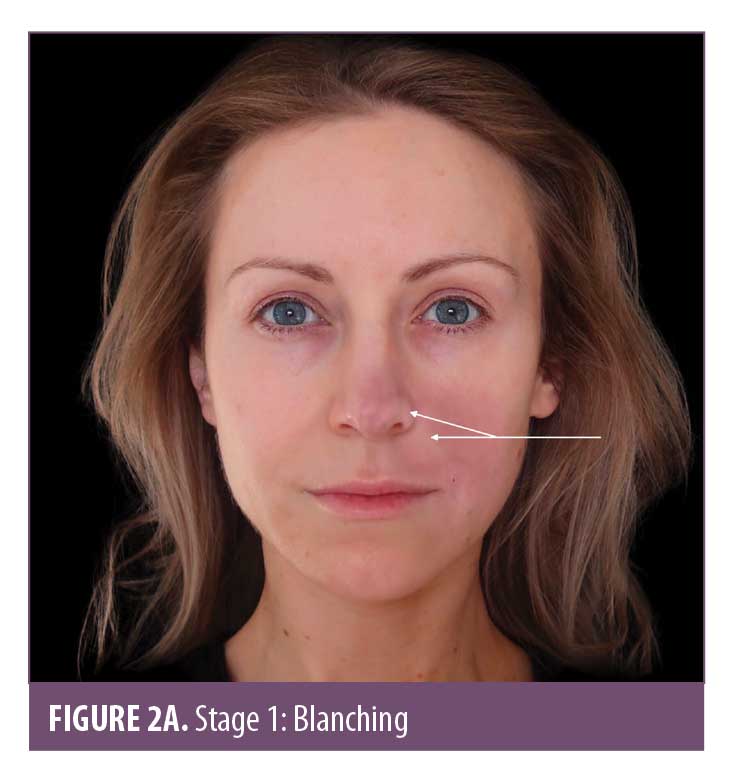
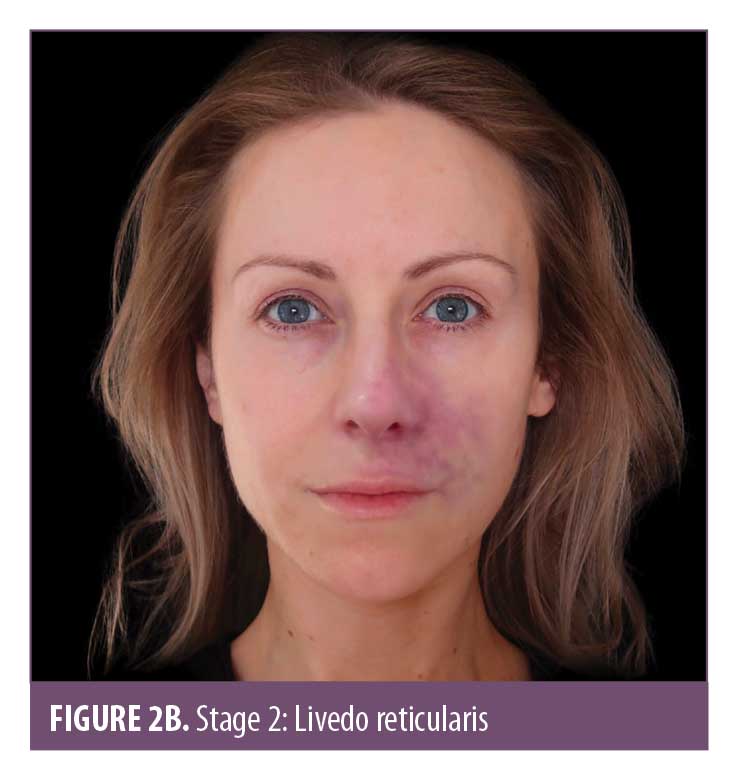
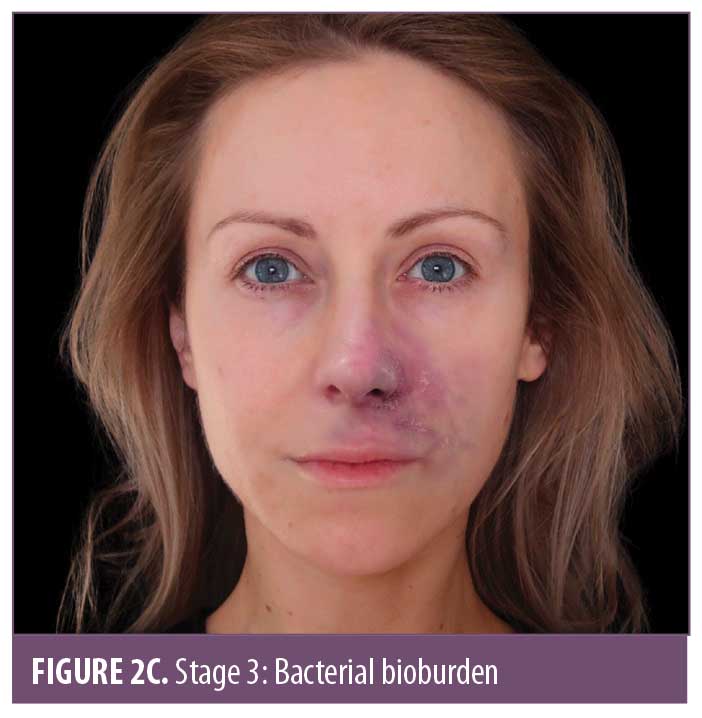
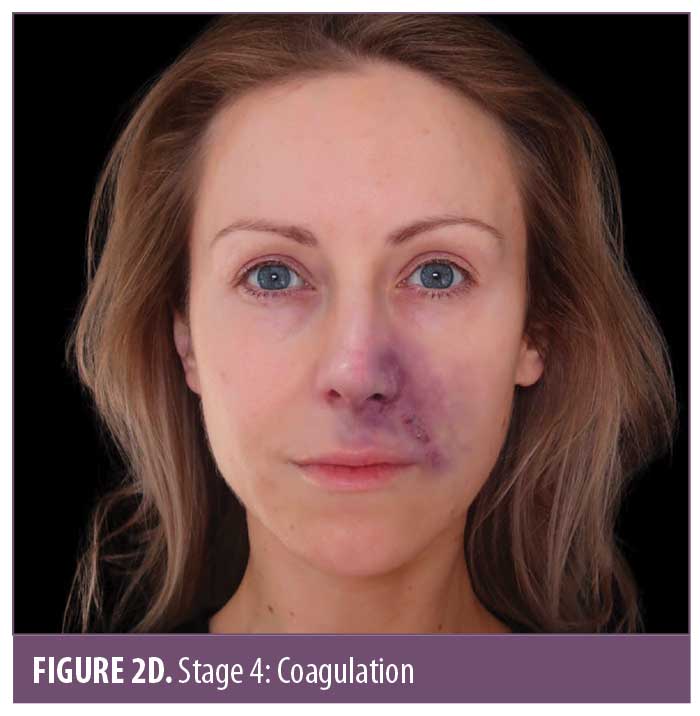
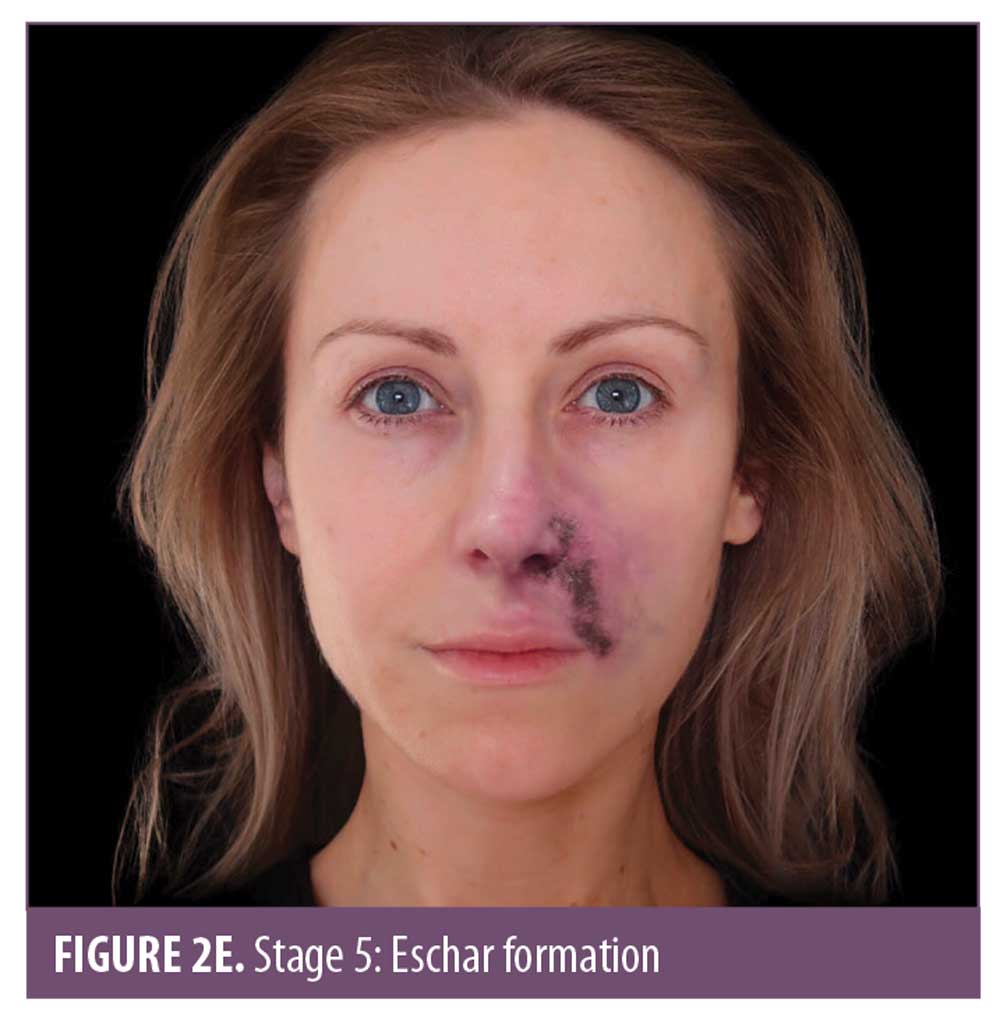
The ischemic skin changes of pallor and reticulation do not indicate inevitable necrosis.15 All tissues can withstand varying periods of ischemia, depending on the tissue type. The extent of tissue damage will depend on both the magnitude and duration of the ischemia. It is possible to re-establish blood flow with very minor damage to the tissues when pallor or a simple reticulated pattern is present.
Necrosis involves cell death, meaning the tissue is no longer viable. Necrotic lesions, therefore, are wounds.15 Ischemic changes after an arterial occlusion can be complex if it is diagnosed late (i.e., days later), with varying stages of ischemia and tissue damage present across the affected area. There may be areas that will quickly recover or require minimal support (Stages 1, 2 and 3) and areas where there is more established necrosis (Stages 4 and 5). The CMAC board advises attempting to dissolve the hyaluronic acid embolus in the occluded vessel, even if there are areas of established necrosis.
If an arterial occlusion is left untreated, the tissue will progress through the stages of ischemia. The extent of tissue damage is dependent upon the occlusion size, underlying anatomy, collateral supply, general vascular integrity, healing ability, and presence of infection.
Assessing the Stages of a Vascular Occlusion
CMAC advises a strategy of skin assessment to establish the extent and identify the stage of ischemic changes. It is useful to understand the underlying pathological mechanisms that give rise to the skin changes, as well as how to assess and differentiate the stages. Where there is tissue breakdown, and therefore, a wound, this requires specific management after the occlusion has been dissolved. The skin changes in an arterial occlusion follow a relatively standardized trajectory that can be broadly categorized as Stages 1 to 5, as outlined in Table 3.
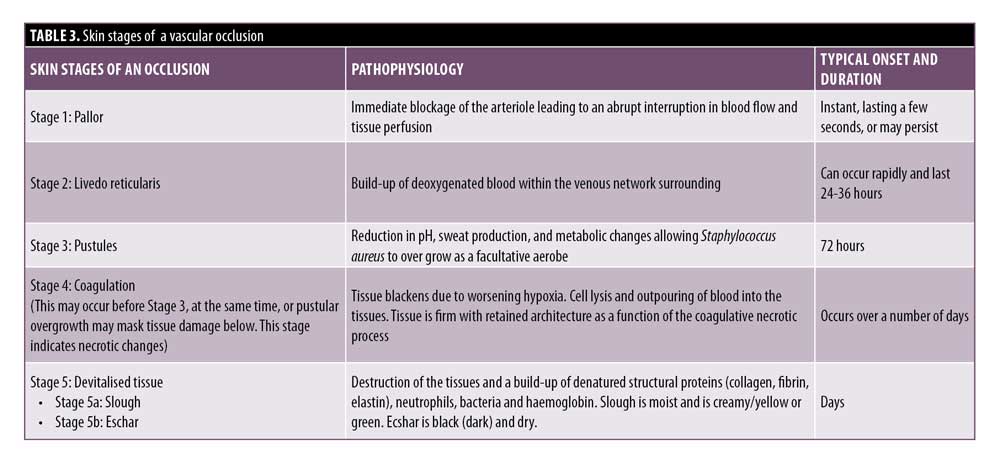
Pallor and subsequent livedo reticularis are a standard phenomenon attributed to arterial compromise, with livedo reticularis being quite typical in appearance due to the anatomy and physiology of the cutaneous microvascular system. Arterial flow to the skin consists of many ascending arterioles rising perpendicularly to the skin surface, which divide to form a capillary bed at the cutaneous surface. The artery is at the center, perfusing the skin in a pattern similar to branches of a tree. Blocking the arteriole or artery arrests or limits blood flow, causing the tissue to “blanch” and display pallor. As time passes, the appearance will evolve, taking on a more mottled effect known as livedo reticularis. Within the vascular subunit, capillary beds are drained of deoxygenated blood at the periphery by the venous plexus which surrounds the central arteriole. Anything that increases the visibility of the venous network (e.g., congestion due to skin hypoxia causing an increase in the volume of deoxygenated blood) causes visibility of these circular plexus formations and, hence, visible livedo reticularis. As the tissue infarcts, it can start to take on a gray hue.16 Livedo reticularis is often described as purple mottling of the skin. The appearance and color will differ depending on the amount of pigment within the tissue. It is important to be able to identify hypoxic signs in skin of color; Figure 3 illustrates the appearance of livedo reticularis occurring in different skin tones.

Stages 3 and 4 may alternate in order, but both indicate that tissue necrosis is developing. The pustular stage seems to be a relatively consistent finding at around Day 3.4 During ischemia, anaerobic metabolism in the tissue prevails.16 There is dysfunction of the sweat glands which reduces sweat production, increasing the pH and reducing the amount of salt on the skin. These factors, combined with the disintegration of normal cellular architecture, allow pathogenic bacteria to overgrow. Meanwhile, normal resident bacteria penetrate deeper into the dermis which provides a warm, moist, oxygen-deficient, nutrient-rich environment where they can thrive.17 Staphylococcus, a normal skin pathogen, is facultative and can thrive in oxygen-reduced environments. It is the immediate cause of bacterial overgrowth.
The darkening of the tissue and the increased blackening of the ischemic area is due to a process called hemorrhagic skin necrosis, which is a manifestation of a thrombotic occlusion of single or multiple blood vessels supplying the skin. The compromised blood vessels leak red cells into the surrounding tissues, where they become trapped within the dying tissue.
The subsequent deoxygenation of hemoglobin in the red blood cells results in the black color. In addition to this process, many pro-inflammatory cytokines are synchronously released by dying cells. At the boundary of the necrotic area, the blood vessels dilate, resulting in hyperemia. This gives rise to the dusky gray-red color of the surrounding skin.18
Some late ischemic insults look like very dark areas of skin prior to any break down of the tissue. Even after hyaluronidase is administered, the tissue in Stages 3 or 4 may break down, as the damage is already done. Necrosis, with retained tissue architecture, is termed coagulative necrosis. In coagulative necrosis, the enzymes that would normally dissolve dead cells become inactive due to the hypoxic injury. The tissue becomes firm and either shrunken or swollen, with retained architecture in the short term. Cellular digestion is principally dependent on heterolysis, which partly explains the late onset of digestion and removal of dead tissues in this type of necrosis.19
Eschar is devitalized tissue composed of dried blood, exudate, and denatured proteins, mainly collagen, elastin, fibrin, and hemoglobin. Eschar covering a wound is undesirable, as it impedes wound healing and creates an optimal, enclosed environment for bacteria to grow.20
Prior to or instead of the development of eschar, slough might be present. Slough is devitalized tissue but differs to eschar in its level of hydration. If the wound is wet, slough can appear as cream, yellow-green, gelatinous/stringy material. If the slough is green, this is a visual indicator of infection. Regardless of whether eschar or slough is present, the wound will need to be managed to avoid infection and to promote optimal healing. Both sequelae indicate necrosis, the extent of which depends on the significance of the occlusion.21
If the skin starts to break down or breaks down after reperfusion with hyaluronidase, it is important to note that necrotic tissue provides a nutrient-rich basis for bacterial proliferation, even if there is a dry eschar appearance. There might be a place for autolytic debridement with topical agents. It is also critical that the wound does not become too “wet” and start producing high volumes of bacterial-rich exudate.17 Any occlusion that has resulted in tissue breakdown requires monitoring and follow up with wound support if needed.
Reperfusion Injury
Tissue damage does not stop at the ischemic insult. Once the vascular supply is re-established, oxygen replenishment prompts enzymatic reactions that produce harmful mediators, including superoxide, hydrogen peroxide, and hydroxyl free radicals.22 These harmful radicals cause damage to the endothelial cells, reduction in nitric oxide (NO) levels and an impairment of neutrophil-mediated bacterial killing. They further induce production of more inflammatory mediators, causing a localized increase in neutrophils. The leukocytes become adherent to the endothelial wall causing vasoconstriction and a low-flow state. Free radicals and the reduction of NO causes vasoconstriction and intravascular thrombus.22 Intervention, therefore, is time sensitive, with prolonged ischemia resulting in a more significant reperfusion injury. When necrosis is established, there will already be a wound presentation and reperfusion may accelerate the breakdown of the tissues. Within other organs (e.g., cardiac tissue), optimal time to reperfusion is known. However, it is less defined within the skin. It is, therefore, important that ischemic changes are promptly managed.15,23
Hyperbaric oxygen therapy (HBOT) is often mentioned in the management of filler-related vascular occlusion and blindness. The level of evidence supporting the use of HBOT in acute filler complications is generally weak. There have, however, been case reports demonstrating efficacy in treating idiopathic cilioretinal artery (CLRA), central retinal vein occlusion, and benefit in treating visual impairment linked to extra ocular muscle ischemia following calcium hydroxyapatite filler injection.24,25 HBOT appeared particularly efficacious in treating visual impairment due to anterior segment ischemia following HA injections and when included in therapy in two cases of skin necrosis.26,27 Zhang28 further reported inclusion of HBOT in treating three patients with visual loss and skin necrosis; all showed improvements in skin healing, but not in terms of sight recovery. HBOT is often included as part of a treatment strategy, but there is a lack of head-to-head data focused on this intervention. Any additional benefit of using HBOT and the stage at which it offers benefit remains unquantified.
Treating a Vascular Occlusion Caused by Cross-linked Hyaluronic Acid
Before the treatment of a vascular occlusion is discussed, it is important to understand the vascular subunits, how they are connected, and how the pattern of ischemic changes dictate where to inject the hyaluronidase. The body is composed of vascular territories called angiosomes (Figure 4) which are distinct in area and supply three-dimensional blocks of tissue, including the skin. Some areas of skin are supplied by a single cutaneous perforator arteriole, which arises from within the angiosomal subunit from the source artery, whereas others are supplied by a single cutaneous perforator supplying several vascular territories via one of two anastomotic connections. True anastomosis are high-pressure vessels maintaining consistent patency and flow; however, choke vessels are low pressure and can collapse to prevent noxious materials passing into adjacent vascular territories.29
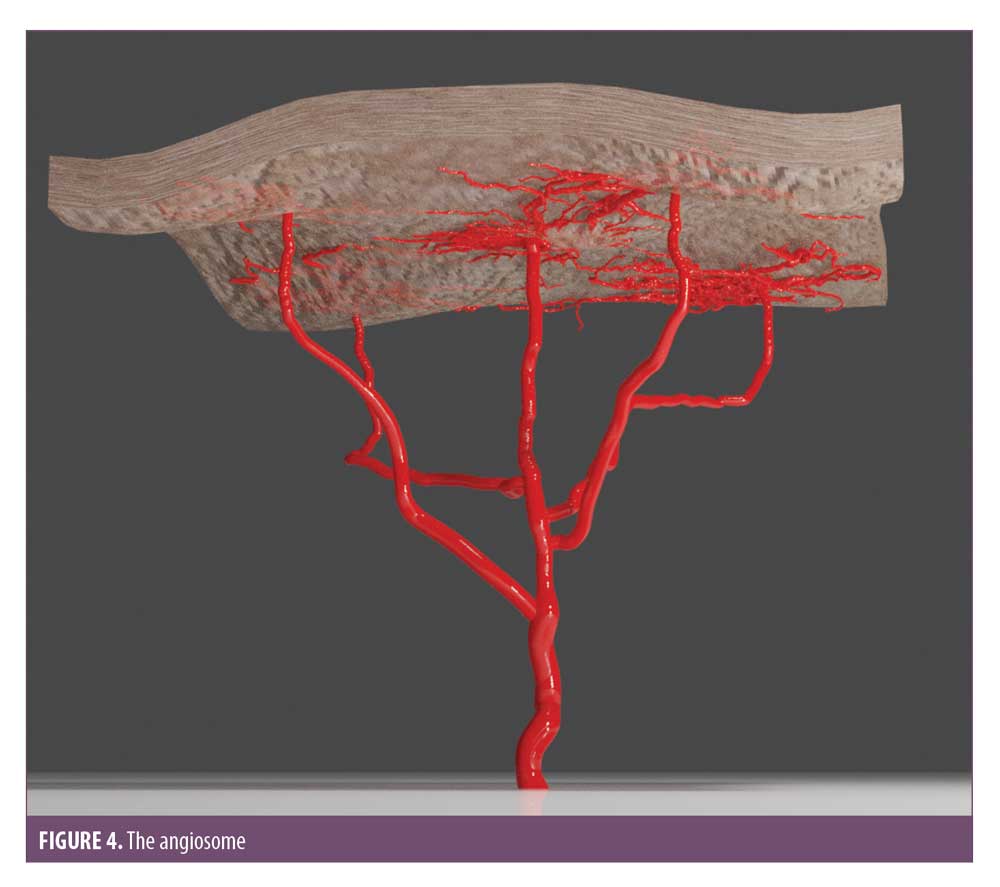
Injecting hyaluronic acid into the main artery or one of its perforators can cause vessel spasm over the entire angiosomal region by collapsing the choke anastomotic connections. It was found that hyaluronic acid produced an irritant response; a noxious stimulus, which can precipitate the collapse of choke vessels.5,28 This explains one of the reasons why ischemia can be found in areas distal to where the injection occurred. In addition to this, turbulent flow within the vessels can cause fragmentation of the hyaluronic acid and an occlusion distal to the injection site. It is imperative that the entire ischemic area is treated to ensure the emboli is dissolved.
The mainstay of treatment is with hyaluronidase, but a study by Spindle et al31 has confirmed that thrombus formation follows injection of intra-arterial HA early on. This supports the need for prompt management to prevent red thrombus accumulation.30 It is important to state that antiplatelets do not dissolve established thrombus; rather, they reduce platelet clumping but do not stop coagulation.31
Immediate Management of Vascular Occlusion
CMAC advises the following management of a vascular occlusion (Table 4). This is based on the authors’ collective experience in treating hundreds of vascular occlusions over the past eight years.
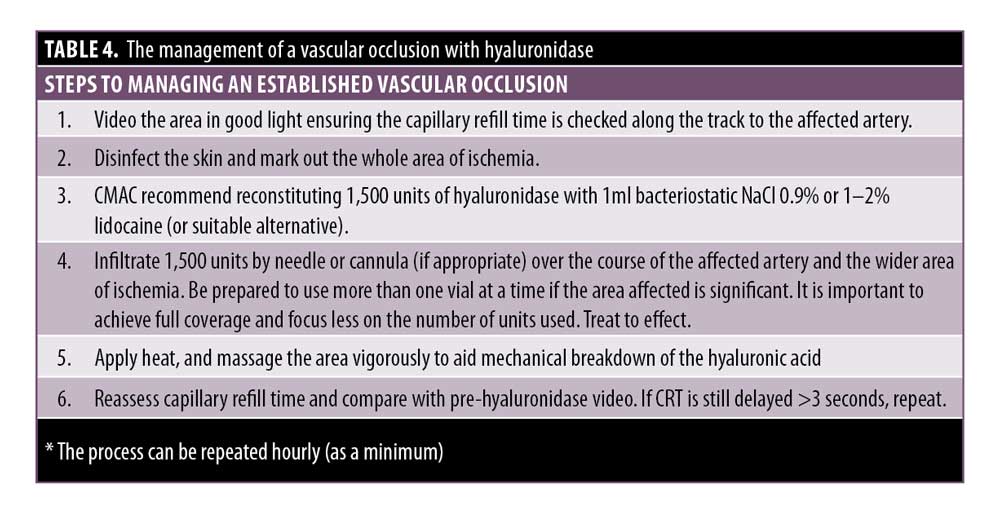
The injection must be stopped immediately if an occlusion is suspected. The patient must be informed of the problem, and it is important to stay calm in the situation. If the clinician does not feel confident to manage the occlusion, seek help from a more experienced colleague to ensure that the issue is dealt with promptly.
CRT must be assessed on both the affected and unaffected side for comparison. A delayed CRT indicates an arterial compromise. A brisk CRT on a background of bluish skin indicates the venous system might be compromised. It is recommended that the CRT is observed prior to treatment to assess what is considered normal for the patient for comparison.
The area must be firmly massaged, applying heat to the area to encourage vasodilation.
If hyaluronidase is required, proceed with the instructions in Table 4.
Hyaluronidase
The use of hyaluronidase in dissolving cross-linked hyaluronic acid is highly effective and has been shown to prevent tissue necrosis.4 Rapid deployment rescues threatened tissue.
CMAC does not recommend performing skin tests in the event of a vascular occlusion, as the risk of anaphylaxis is minimal and there is no recognized validated test concentration to accurately assess Type 1 hypersensitivity (See CMAC guideline for hyaluronidase). As with the administration of any drug in a clinic setting, it is important to have a management plan in place. This should include a stock of adrenaline in the event of anaphylaxis.
CMAC recommends using 1500 units per 1mL, if available, as a reconstitution volume and employing a high-dose pulsed model of dosing.4 Hyaluronidase causes rapid spreading of subcutaneously injected agents. The rate of diffusion is proportional to the amount of enzyme, with the extent being proportional to the volume of fluid.32 To ensure the affected area is exposed to adequate amounts of the enzyme, high concentrations within the affected area are required. It has been evidenced, in animal models, that residual hyaluronic acid has been found in the vessel lumen after submerging in hyaluronidase for one hour.33 This evidences the potential need to redose at one hour. The clinician can choose to reconstitute the hyaluronidase with lidocaine 1% or 2% or another local anesthetic agent which is chemically compatible and also causes some vasodilation.34 There is no need to direct the injection of hyaluronidase into the artery itself, as hyaluronidase readily diffuses into the artery after extravascular injection.4
Management of a Vascular Occlusion Using Ultrasound
Using ultrasound as a method of identifying and effectively dissolving an intra-arterial embolus is gaining momentum. Without ultrasound, effective management of ischemia relies on clinical observation where the precise location of the embolus is not known. The arterial vasculature is complex, and the presence of arterial branches and anastomotic connections mean the filler embolus can travel away from the site of injection.35 Cross-linked hyaluronic acid present within a vessel can cause choke vessels to shut down, causing vascular compromise to other angiosomal regions.29 As a result of these factors, the area of ischemia can be large and/or evolving. Treating complex vascular occlusions can require hours of repeated administration of hyaluronidase. This can result in pain, swelling, bruising, and patient distress. Treatment is often abandoned until the next day to give the patient respite.
High-frequency ultrasound gives the ability to visualize location, depth, and size of the filler embolus. Publications by Schelke et al35 have indexed many cases where a cross-linked hyaluronic acid embolus is effectively dissolved using an average of 35 to 60 units of hyaluronidase, using up to 150 units in a small number of cases.35
The ability to identify and effectively treat a vascular occlusion requiring a small amount of hyaluronidase while being assured of restored perfusion cannot be understated. Ultrasound enables effective management with minimal patient discomfort, swelling, and distress.
Hopefully, the use of ultrasound as a diagnostic tool will continue to gain importance in the field of aesthetic medicine.
Further Pharmacological Management
There is no direct evidence that aspirin prevents platelet aggregation in the event of a hyaluronic acid-related occlusion. However, it is reasonable, if safe to do so, based on the an extrapolation of the evidence from acute coronary syndrome, to prescribe 300mg as a stat dose with 75mg daily until the occlusion is dissolved and the tissue has been reperfused. If the patient is allergic to aspirin, clopidogrel at a dose of 300mg stat, then 75mg daily can be used.36
Nitroglycerin paste and hyperbaric oxygen are not evidence-based ancillary therapy for cross-linked hyaluronic acid filler in early management of vascular compromise, but they are still recommended for the treatment of particulate filler vascular compromise, as all measures should be undertaken to reverse compromise.37 Clinicians can treat these hyaluronic acid filler complications with topical nitroglycerin paste based on the knowledge that topical nitroglycerin causes vasodilation. In filler-induced tissue ischemia, however, filler product is present within arterioles. Theoretically, applying nitroglycerin paste early might not improve perfusion and could worsen ischemia with dilation of vessels and further propagation of product into the smaller arterioles and capillaries.38
Given the lack of evidence and risk of venous congestion to the area, CMAC does not recommend nitroglycerin paste. Effective wound care and management is critical in the time after 48 hours or once the skin has begun to blister (approximately 72 hours postinjection). CMAC advises stringent wound support to optimize healing.36
CMAC would urge clinicians to avoid using steroids routinely unless there is a clinical indication. Wound management and infection prevention is of paramount importance and giving steroids can compromise wound healing or worsen any existing, early infection.
Sildenafil is often suggested for the management of vascular occlusion. However, there are no head-to-head studies measuring outcomes and efficacy of this intervention. Sildenafil and tadalafil have been linked with central serous chorioretinopathy and phosphodiesterase- 5 inhibitors can also cause drops in blood pressure, especially in combination with oral nitrates.39,40 Prescribing of sildenafil should be a considered decision, and CMAC advises against using it in the management of a vascular occlusion. In addition, oral antivirals should only be given if the tissue has started to break down.
In the event the tissue has broken down or starts to break down, it is important to prevent infection and support optimal wound healing. Debriding, application of topical agents, and the use of antibiotics might be required to prevent further complications. However, not all wound scenarios require debridement or mechanical removal of the tissue. There are useful dressings and topical agents that support optimal wound healing and can also promote autolysis when required. The use of antibiotic agents should not be routine and used only if clinically indicated.
Ensure contemporaneous notes are taken, including images. It is also advised that insurers are contacted to make them aware of the situation. Vascular occlusions are a distressing situation that may lead to claims.
Patient follow-up. All patients should be educated on the warning signs of a vascular occlusion and be provided with emergency contact details should any of these signs present or they have concerns. In the event of a suspected vascular occlusion, an urgent face-to-face assessment must be scheduled.
Regardless of the clinical severity, review and monitoring is required. Vascular occlusions that are treated promptly to clinical resolution still require follow up the next day to ensure no deterioration has occurred. More significant occlusions causing skin breakdown must be followed up and managed until the area has healed.
Acknowledgments
All illustrations in this article (Figures 1–4) were created by Dr. Toni Burke. Complications in Medical Aesthetics Collaborative (CMAC ) is an organization set up to provide support and education to medical aesthetic clinicians. Members are part of a collaboration, and through working with the membership, CMAC aims to capture data to help improve patient safety. For more details, please see https://www.cmac.world/.
References
- Belezney K, Carruthers J, Humphrey S, et al. Update on avoiding and treating blindness from fillers: A recent review of the world literature. Aesthet Surg J. 2019;39(6):662–674.
- Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinol tolerance time. Opthalmology. 1980;87(1):75–78.
- Tobalem S, Scultz JS, Chronopoulos A. Central retinal artery occlusion-Rethinking retinal survival time. BMC Ophthalmol. 2019;18(1):101.
- Delorenzi C. New High Dose Pulsed Hyaluronidase Protocol for Hyaluronic acid filler vascular events. Aesthet Surg J. 2017;37(7):814–825.
- Taylor GI, Shoukath S, Gascoigne A, et al. The functional anatomy of the opthalmic angiosome and itsimplications in blindness as a complication of cosmetic facial filler procedures. Plast Reconstr Surg. 2020;146(4):745.
- Goodman GJ, Magnusson MR, Callan P et al. A consensus on minimising the risk of hyaluronic acid embolic visual loss and suggestions for immediate bedside management. Aesthet Surg J. 2020;40(9):1009–1021.
- Zhou SB, Chaing, MD, Liu, K. False sense of safety: blunt cannulas cause the majority of severe vascular complications in hyaluronic acid injection. Plast Reconstr Surg. 2020; 146(2):240e–241e.
- Casabona G. Blood aspiration test for cosmetic fillers to prevent accidental intravascular injection in the face. Dermatol Surg. 2015;41(7):841–847.
- Van Loghem J. Sensitivity of aspiration as a safety test before injection of soft tissue fillers. J Cosmet Dermatol. 2018;17:39–46.
- Torbeck RL, Schwarcz R, Hazan E, et al. In vitro evaluation of preinjection aspiration for hyaluronic fillers as a safety checkpoint. Dermatol Surg. 2019;45(7):954–958.
- Kapoor KM, Kapoor P, Heydenrych I, Bertossi D. Vision loss associated with hyaluronic acid fillers: a systematic review of the literature. Aesth Plast Surg. 2020;44(3): 929–944.
- Buhren BA, Schrumpf H, Bölke E, et al. Standardized in vitro analysis of the degradability of hyaluronic acid fillers by hyaluronidase. Eur J Med Res. 2018;23(1):37.
- Kapoor KM, Murthy R, Hary SLA, et al. Factors influencing pre-injection aspiration for hyaluronic acid fillers: A systematic literature review and metanalysis. Dermatol Ther. 2020:e14360.
- Urdiales-Gálvez F, Delgado NE, Figueiredo V, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42(2):498–510.
- Kalogeris T, Baines G, Krenz M, Korthuis R. Cell biology of ischaemia/reperusion injury. Int Rev Mol Biol. 2012;298:229–317.
- Gibbs M, English J, Zirwas M. Livedo reticularis: An update. J Am Acad Dermatol. 2005;(52):1009–1019.
- World Union of Wound Healing Societies (2008) Medical Education Partnership Ltd, London. Wound Infection in Clinical Practice: An International Consensus. Wounds International website. Published October 14, 2009. https://www.woundsinternational.com/resources/details/wound-infection-clinical-practice-wuwhs-international-consensus.
- Patel GK. How to diagnose and treat a hemorrhagic skin necrosis. Wounds UK. 2007;3(4).
- Adigun R, Basit H, Murray J. Cell Liquefactive Necrosis. [Updated 2020 Aug 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430935/
- Thomas AM, Harding KG, Moore K. The structure and composition of chronic wound eschar. J Wound Care. 1999;8(6):285–287.
- White W, Asimua M. Chapter 8: Assessment and management of non-viable tissue. In: Swanson T, Asimus M, McGuinness B, eds. Wound Management for the Advanced Practitioner. 1st ed. IP Communications, 2014: 170–212.
- Al-Quattan MM, Al-Kattan WM. Skin wound healing, ischemia-reperfusion injury, and nerve rejeneration: Similarities in the sequential events and molecular basis. Can J Plast Surg. 2004;12(3):131–133
- McGarr GW, Hodges GJ, Mallette MM, Cheung SS. Ischemia-reperfusion injury alters skin microvascular responses to local heating of the index finger. Microvasc Res. 2018;118:12–19.
- Celebi AR, Kilavuzoglu AE, Altiparmak UE, et al. Hyperbaric oxygen for the treatment of the rare combination of central retinal vein occlusion and cilioretinal artery occlusion. Diving Hyperb Med. 2016;46(1):50–53.
- Sung WI, Tsai S, Chen LJ. Ocular complications following cosmetic filler injection. JAMA Ophthalmol. 2018;136(5):e180716.
- Worley N, Lupo M, Holcomb K, et al. Hyperbaric oxygen treatment of keratitis following Facial hyaluronic acid injection. Ochsner J. 2020;20(2):193–196.
- Darling MD, Peterson JD, Fabi SG. Impending necrosis after injection of hyaluronic acid and calcium hydroxylapatite fillers: report of 2 cases treated with hyperbaric oxygen therapy. Dermatol Surg. 2014;40:1049–1052.
- Zhang L, Pan L, Xu H, et al. Clinical observations and the anatomical basis of blindness after facial hyaluronic acid injection. Aesthetic Plast Surg. 2019;43(4):1054–1060.
- Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg. 1987;40(2):113–141.
- Baley-Spindel I, Villasenor-Villalpando E, Marquez-Espriella C, et al. Perivascular hyaluronidase with alteplase as treatment for hyaluronic acid thrombus. Aesthet Surg J. 2019;40(5):551–559.
- Mir AI, Ali N, Kohli A et al. Role of the antiplatelet drug in deep vein thrombosis. J Med Sci. Clin Res. 2017;5(10):28689–28695.
- Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31: 893–897.
- Rauso R, Zerbinati N, Fragola R, Nicoletti GF, Tartaro G. Transvascular hydrolysis of hyaluronic acid filler with hyaluronidase: an ex vivo study. Dermatol Surg. 2020;00:1–4.
- Summary of product characteristics- Hyaluronidase. Wockhardt UK LTD. Accessed via (Medicines.org.uk/emc/product/1505) on 02/12/20. [Date of last revision 19/2/2015]
- Schelke LW, Velthuis P, Kadouch J, Swift A. Early ultrasound for diagnosis and treatment of vascular adverse events with hyaluronic acid fillers. J Am Acad Dermatol. 2019;S0190–9622(19)32392–8.
- Coronary syndromes in adult: NICE quality standard [QS68]. National Institute for Health and Care Excellence website. Published September 2014. Updated November 18, 2020. https://www.nice.org.uk/guidance/qs68.
- Graivier MH, Bass LM, Lorenc ZP, et al. Differentiating Nonpermanent Injectable Fillers: Prevention and Treatment of Filler Complications. Aesthet Surg J. 2018; 6(38): S29–S40.
- Hwang CJ, Morgan PV, Pimentel A, et al. Rethinking the role of nitroglycerin ointment in ischemic vascular filler complications: An animal model with ICG imaging. Ophthalmic Plast Reconstr Surg. 2016;32(2):118–122.
- Kaye R, Chandra S, Sheth J, et al. Central serous chorioretinopathy: An update on risk factors, pathophysiology and imaging modalities. Prog Retin Eye Res. 2020;79:100865.
- Summary of product characteristics- Sildenafil. Pfizer LTD. Accessed via (Viagra 100 mg film-coated tablets – Summary of Product Characteristics (SmPC) – (emc) (medicines.org.uk)) on 02/12/20. [Date of last revision 11/2020].