 J Clin Aesthet Dermatol. 2021;14(5):40–45.
J Clin Aesthet Dermatol. 2021;14(5):40–45.
by Khaled Gharib, MD; Abdalla Kandil, MD; Ayman Marie, MD; and Hanim Mounir, MSc
Drs. Gharib and Kandil, and Mr. Mounir are with the Dermatology Department, Faculty of Medicine at Zagazig University in Zagazig, Egypt. Dr. Marie is with the Microbiology Department, Faculty of Medicine at Zagazig University in Zagazig, Egypt.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background: Lichen planus is a multifactorial chronic inflammatory disease with diverse clinical manifestations involving the skin, hair, nails, and mucous membranes.
Objective: We evaluated the clinical efficacy and adverse effects of Bacillus Calmette-Guérin polysaccharide nucleic acid (BCG-PSN) injection, vitamin D injection, and the combination of both in the treatment of different types of lichen planus.
Methods: Thirty patients ranging in age from 12 to 70 years with clinically diagnosed lichen planus were enrolled in this study and divided into three groups according to treatment regimen.
Results: A partial therapeutic response in three patients and no improvement in seven patients were achieved in the intramuscular vitamin D injection group. Meanwhile, the intralesional injection of BCG-PSN was associated with complete response in three patients, partial response in three patients, and no response in four patients. The combination of both intralesional injection of BCG-PSN and intramuscular injection of vitamin D was associated with complete response in two patients, partial response in four patients, and no response in four patients.
Conclusion: BCG-PSN alone appears to be efficacious in the treatment of lichen planus, and vitamin D in combination with BCG-PSN had no effect on cutaneous lichen planus.
Keywords: Lichen planus, Bacillus Calmette-Guérin, vitamin D
Lichen planus (LP) is an inflammatory disease that affects the skin, hair follicles, mucous membranes, and nails.1 Cutaneous LP is characterised by flat-topped, polygonal, violaceous papules and plaques, which can cause intense itching. Lesions are usually seen on the flexor areas of the upper extremities, genital area, and mucous membranes.2 Clinical data suggest that there are relationships between LP and a number of exogenous factors (e.g., viruses, drugs, and contact allergens).3 LP is a disease associated with autoimmune destruction of basal keratinocytes by CD8+ lymphocytes.4
The cause of LP is unknown: it is not contagious and involves no known pathogen; as such, it is thought to be a T-cell-mediated autoimmune reaction (in which the body’s immune system targets its own tissues). This autoimmune process triggers apoptosis of epithelial cells. Several cytokines are involved in LP, including tumor necrosis factor alpha, interferon gamma, interleukin (IL)-1 alpha, IL-6 and IL-8. This autoimmune T-cell-mediated process is thought to be in response to some antigenic change in the oral mucosa, but a specific antigen has not yet been identified.5
Third-generation Bacillus Calmette-Guérin (BCG) extract with various immunologically active materials, including polysaccharides and nucleic acid, has the ability to regulate the subsets of T-cells (CD4 and CD8 cells) and subtypes of helper T-cells (Th1 and Th2). Topical BCG with polysaccharide nucleic acid (BCG-PSN) therapy has been reported in the successful management of certain dermatologic diseases with good curative effects and few side effects and toxicities.6
The immunosuppressant and immunomodulation properties of vitamin D are already established. Vitamin D acts on both B and T lymphocytes. The expression of vitamin D receptors (VDRs) in immune cells such as activated T- and B-cells highlights the role of vitamin D in the modulation of various types of immunity. VDR signaling can modulate the innate and adaptive immunity. It is an established fact that LP is an immune-related condition.7
The aim of this study was to evaluate the clinical efficacy and adverse effects of BCG-PSN, vitamin D, and their combination in the treatment of different types of LP.
Methods
Patients. Thirty patients ranging in age from 12 to 70 years with clinically diagnosed LP were enrolled in this study conducted at the Outpatient Clinics of the Department of Dermatology, Venereology, and Andrology at Zagazig University Hospital from May 2018 to May 2019. This study was approved by the institutional review board (no. 4546/4-4-2018) of the Faculty of Medicine, Zagazaig University, and written informed consent to participate in the study was obtained from all participants.
The diagnosis of each patient was made on the basis of history and clinical presentation. Patients were distributed into three groups as follows: Group A included 10 patients with LP treated by an intramuscular injection of an ampoule of vitamin D (200,000 IU) every two weeks for one month, Group B included 10 patients with LP who were treated by an intralesional injection of 0.5mL of BCG-PSN once weekly for four weeks, and Group C included 10 patients with LP who were treated by an intralesional injection of 0.5mL of BCG-PSN once weekly for four weeks and an intramuscular injection of an ampoule of vitamin D (200,000 IU) every two weeks for one month.
Immunocompromised patients or those with severe oral mucous disease, such as pemphigus and Stevens–Johnson syndrome; those on immunosuppressive therapy during the previous three months; those using topical treatments within a week before the study; those with a bleeding tendency; those with active viral, bacterial, or fungal infections; those with fever; those with a generalized rash; and those with a history of allergy to BCG as well as pregnant or lactating women were excluded from this study.
Each patient was subjected to the following:
- Complete history-taking, including personal history, including name, age, sex, occupation, and residence; history of dermatological disease, including onset, course, duration, site and history of previous treatment for the disease; history of other dermatological or systemic diseases; and family history
- A general examination of body systems to discover associated medical conditions.
- Detailed dermatological examination, including a description of the LP lesions, including site, number, type and size; unvolvement of mucous membrane, hair, and nails; and ny associated other dermatological diseases
- Laboratory investigations, including complete blood cell count, anti-hepatitis C virus (HCV) antibody, random blood sugar, and liver function tests
- Photographs taken before, during, and after treatment
Materials. The BCG-PSN was extracted from BCG at the Immunology Research Lab, Department of Microbiology and Immunology, Zagazig University, according to the following steps. First, one lyophilized vial of BCG (Vacsera, Dokki, Egypt), with each 0.1mL containing between 1×105 and 33×105 colony forming unist (CFUs), was reconstituted in an aliquot of 1mL of saline. Then, 1mL was transferred to a sterile 1.5-mL Eppendorf tube and kept at 4°C for 15 minutes . Next, lysozyme (final concentration of 10mg/mL) was added, and the tube was incubated at 4°C for 30 minutes; then, proteinase K (final concentration of 100µg/mL; Qiagen, Hilden, Germany) was added, and the tube was incubated at 56°C for five hours. The tube was then heated at 95°C for 10 minutes to inactivate the proteinase K and cooled at 4°C for not more than five hours. The sample was next centrifuged at 12,600g for five minutes, the supernatant was removed to a new 1.5-mL Eppendorf tube, and 1mL of saline was added. The final preparation of BCG-PSN was a colorless liquid. Notably, this method of preparation is different from that used in China; it did not involve the use of phenol, which may induce several side effects, including hypersensitivity, skin necrosis, and central nervous system toxicity.8
Separately, the vitamin D used in this study was an ampoule of cholecalciferol (200,000 IU).
Treatment. As mentioned, Group A was treated with one ampoule of vitamin D (200,000 IU) every two weeks for one month. Group B was treated with four intradermal injections of BCG-PSN (0.5 mL) once weekly for four weeks. The BCG-PSN was injected using an insulin syringe with a 0.5-in, 30-gauge needle as a 0.5-mL single injection in the center of the lesion. Pressure with sterile gauze was applied for three to five minutes to avoid bleeding from the site of injection. Finally, Group C was treated with four intradermal injections of BCG-PSN (0.5 mL) once weekly for four weeks and one intramuscular injection of an ampoule of vitamin D (200,000 IU) every two weeks for one month.
Injections were discontinued if the lesion disappeared or side effects occurred. Photographs were taken of each patient at every visit using a camera phone to compare and evaluate the clinical response.
The responses of cutaneous LP were evaluated on the basis of 1) lesion improvement, classified as complete response (complete disappearance of lesions and pruritus), partial response (more than 50% clearance of lesions and no or mild residual pruritus), or no response (less than 50% clearance of lesions and/or residual significant pruritus); and 2) pruritus severity, classified as either no pruritus, mild pruritus, or significant pruritus.
Results
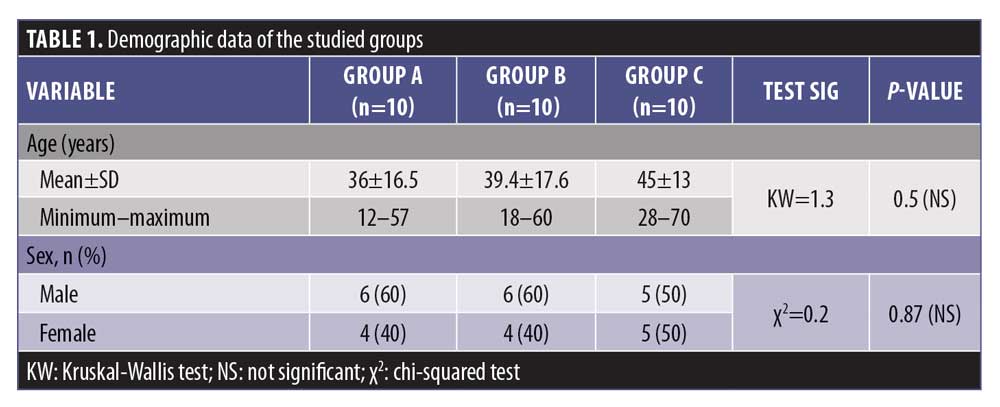
The mean age of Group A was 36 years, that of Group B was 39.4 years, and that of Group C was 45 years. There was a statistically insignificant difference in age and sex between the studied groups (p>0.05) (Table 1).
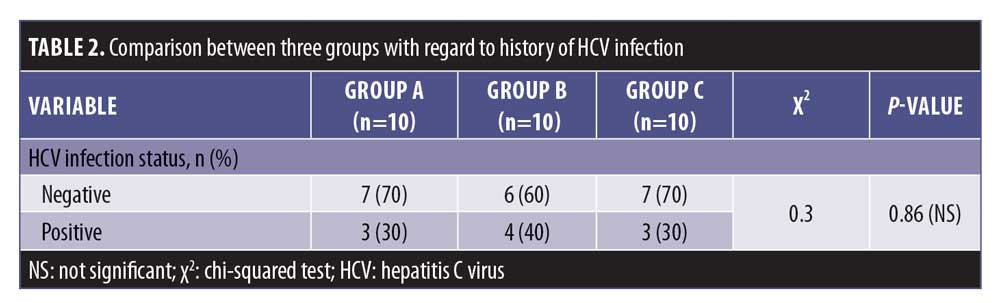
There was a statistically insignificant difference between Group A and Groups B and C regarding the percentage of previously treated patients (p>0.05), whereas there was a statistically significant difference between Group B and Group C in terms of the percentage of previously treated patients (p<0.05) (Table 2).
The mean duration of disease in Group A was 10.3 months, that in Group B was 32.6 months, and that in Group C was 7.6 months. There was a statistically significant difference (p<0.05) (Table 3).
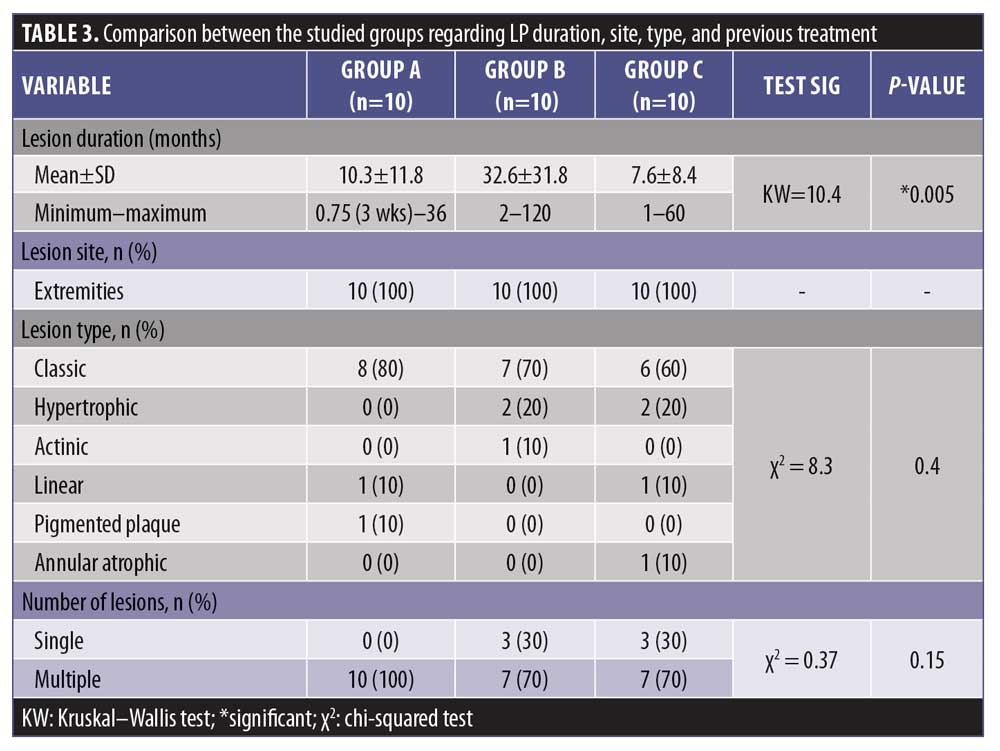
There was a statistically significant difference between Group A and Group B regarding the duration of disease (p<0.05). Also, there was a statistically significant difference between Groups B and C regarding the duration of disease (p<0.05), whereas there was a statistically insignificant difference between Groups A and C regarding the duration of disease (p>0.05).
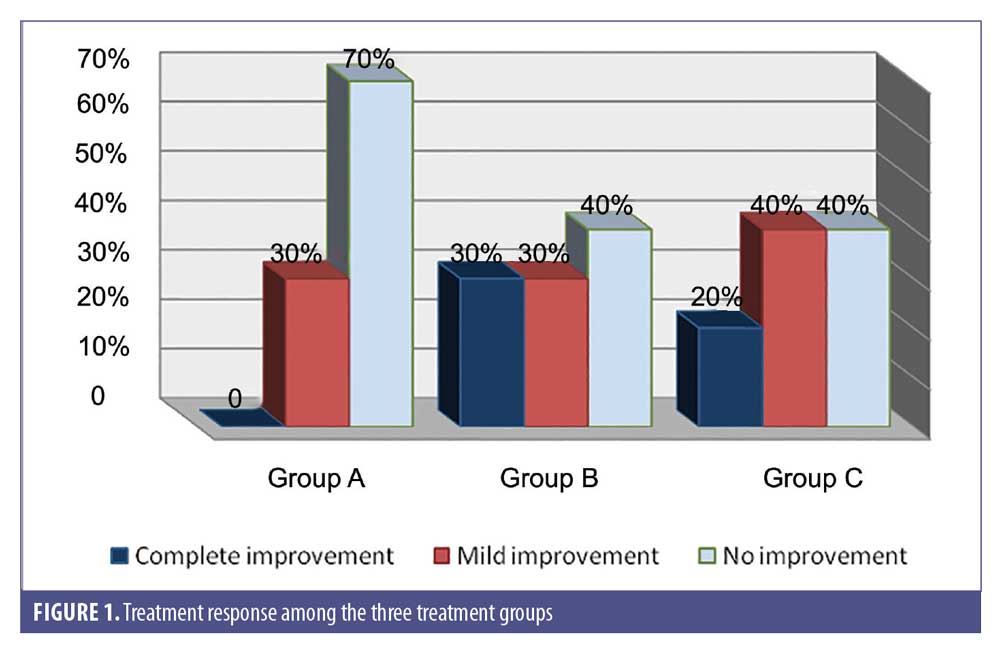
There was complete improvement in 20 percent of Group C, 30 percent of Group B, and in zero percent of Group A; the difference between the groups was statistically insignificant (p>0.05) (Figure 1).
In Group B, 50 percent of patients had an ulcer, while no one in Group A or Group C had an ulcer as a side effect related to treatment. This difference was statistically significant (p<0.05) (Table 4).
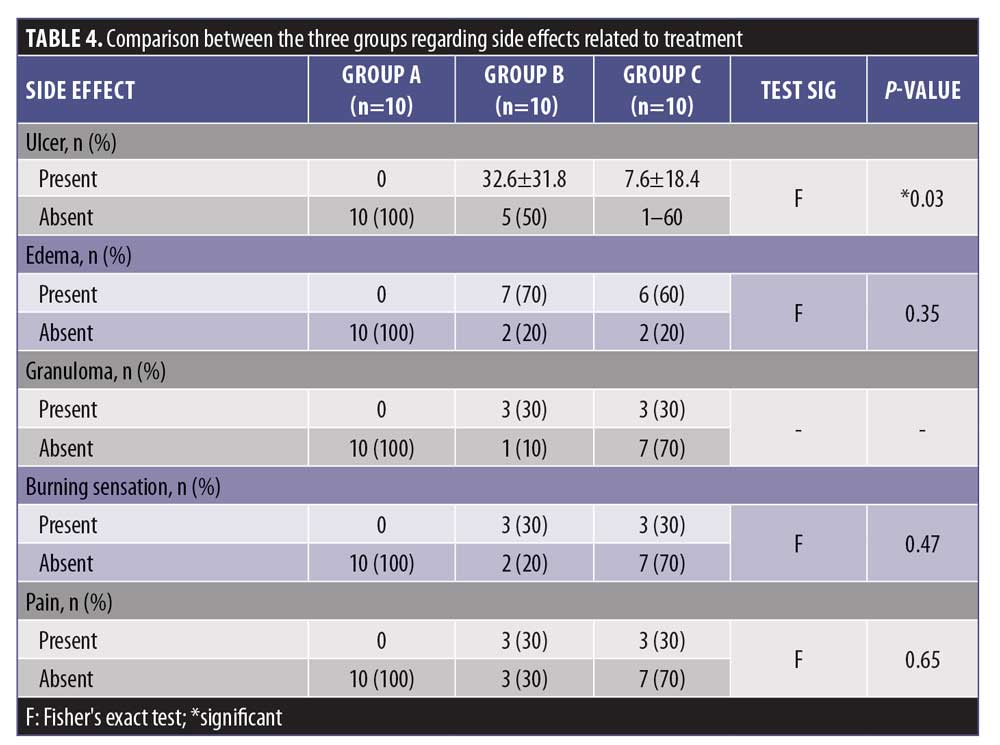
In Group B, 90 percent of patients experienced treatment side effects, while 60 percent of Group C experienced side effects. No patient in Group A had side effects related to treatment. The difference between groups was statistically significant (p<0.05).
There was a statistically insignificant difference between Groups B and C (p>0.05) with regard to the percentage of side effects (Figure 2 and Table 5).
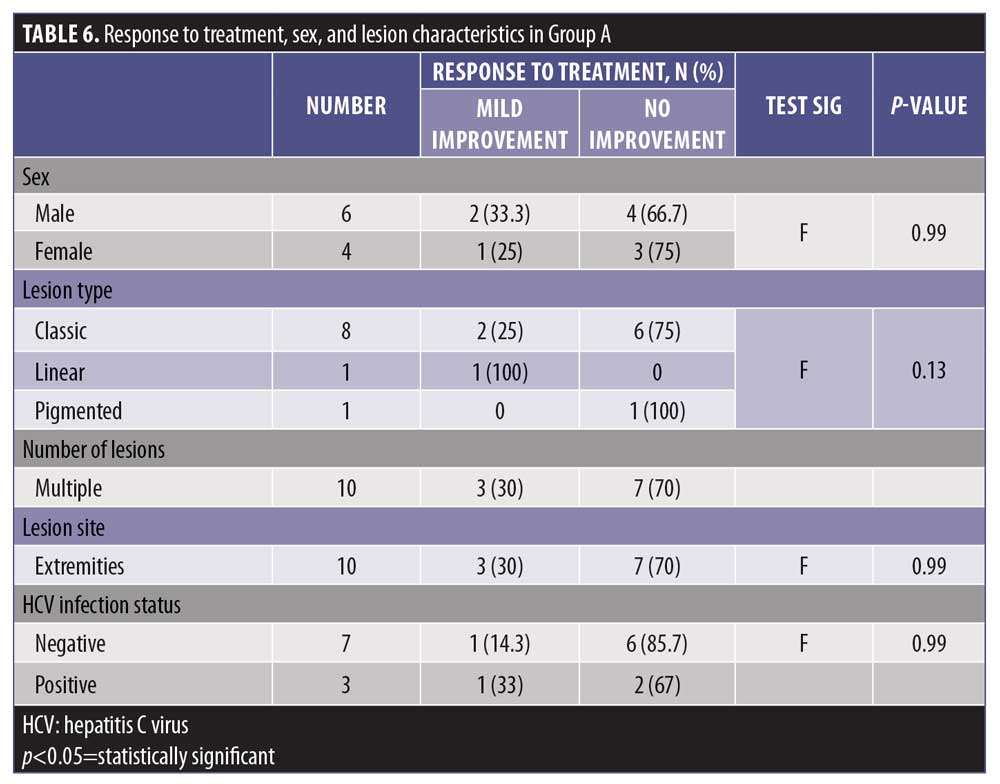
The level of response to treatment in group A was statistically insignificant with regard to sex and lesion characteristics (p>0.05) (Table 6).
Among men, 50 percent experienced complete improvement and 50 percent showed no improvement, while 75 percent of women experienced partial improvement and 25 percent had no improvement. The difference according to sex was statistically significant (p<0.05) (Table 7).
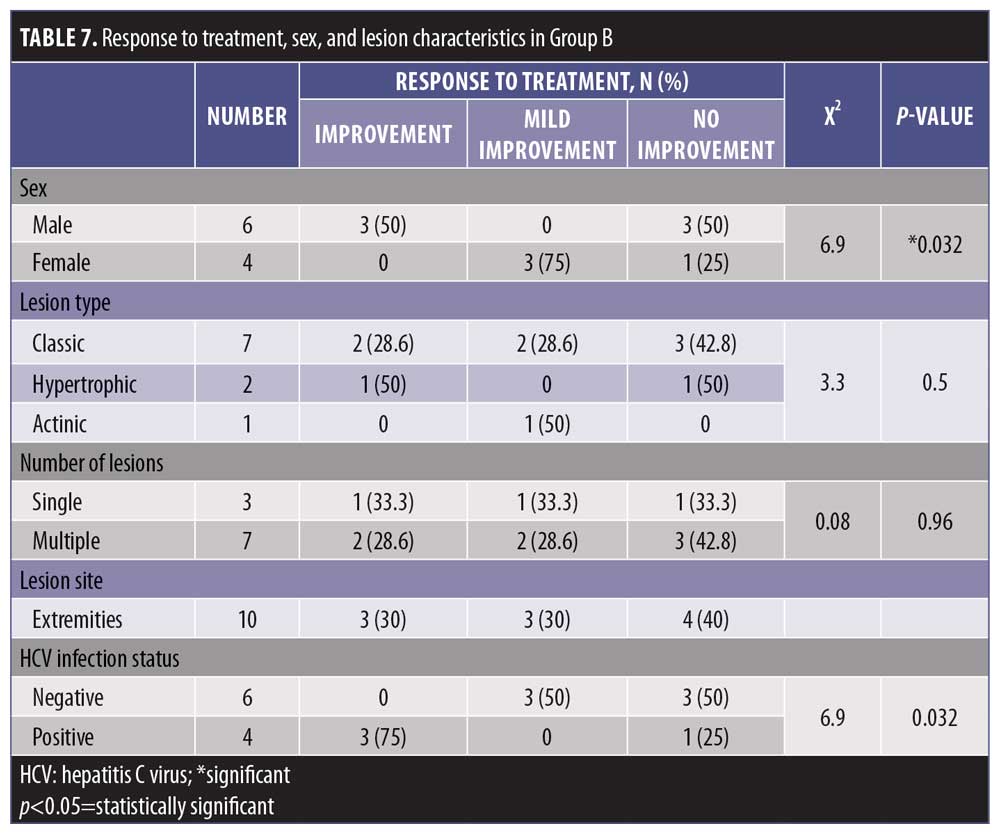
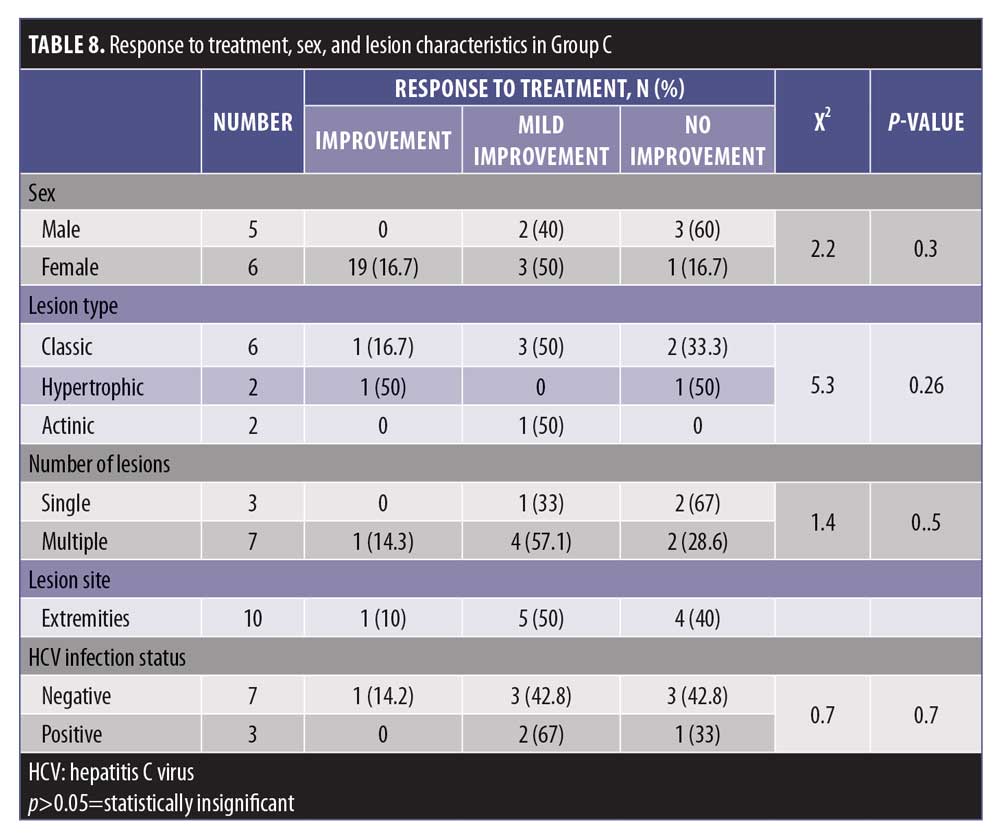
The level of response to treatment in group C was statistically insignificant with regard to sex and lesion characteristics (p>0.05) (Table 8).
Discussion
LP is a chronic inflammatory, mucocutaneous, idiopathic disorder that can affect the oral mucosa through various clinical forms, ranging in appearance from asymptomatic, white, reticulated lesions to ulcerated, erosive and extremely painful ones.9,10
In our study, we examined the clinical efficacy and adverse effects of BCG-PSN, vitamin D, and their combination in the treatment of different types of LP.
A total of 30 patients with clinically proved LP were enrolled in this study, aged from 12 to 70 years. Among the study population, there were 13 women (43.3%) and 17 men (56.7%), who were divided into three groups.
Group A was treated with vitamin D; 70 percent of patients showed no improvement and 30 percent showed mild improvement. Group B was treated with BCG-PSN and 30 percent of patients showed complete improvement, 30 percent showed mild improvement, and 40 percent showed no improvement. Finally, Group C was treated with the combination of BCG-PSN and vitamin D, and 20 percent of patients showed complete improvement, 40 percent showed mild improvement, and 40 percent showed no improvement.
There were no adverse effects in group A (0%). but the adverse effects were significant in Groups B and C. Pain was reported in both Group B (30%) and Group C (50%), whereas a burning sensation occurred in Group B (20%), but did not occur in Group C. Edema occurred in group B (20%) patients and in five patients in Group C, and the difference was statistically significant. This swelling developed within 24 hours after injection but resolved spontaneously within 1 to 2 weeks.
The results of our study indicate that patients given intramuscular vitamin D had no complete response or only mild improvement (30%). This might be due to the low dose of vitamin D and the short duration of the study.
No previously published studies used systemic vitamin D injection for cutaneous LP. However, is vitamin D deficiency a predisposing factor?11 In one case study, vitamin D supplementation administered to patients with oral LP (OLP) resulted in improvements of signs of the disease. The difference in the results might be due to our study only including patients with cutaneous LP.
The results of the present study showed that intralesional injection of BCG-PSN was associated with complete response in 30 percent of studied patients. A higher percentage (87.5%) was reported by Xiong et al,12 who examined the effect of intralesional BCG-PSN injection in the treatment of erosive OLP. Their study showed such might be therapeutically beneficial for erosive OLP recalcitrant to classical treatments.
Xiong et al12 reported the occurrence of side effects in the form of a burning sensation in three of 31 BCG-PSN-treated patients (9.7%). This variability might be due to the relatively smaller number of patients included in our study. Thus, in further studies, it is recommended to increase the number of participants, as granuloma occurred in only one patient in Groups B and C.
Similarly, Metwalli et al13 have also reported a much higher response with intralesional BCG-PSN for OLP than that reported in our study (98.2% vs. 30%).
Also, the study by Nasr et al14 examined BCG-PSN in the treatment of cutaneous LP and OLP. In the cutaneous LP group, complete response was apparent in 76.9 percent and 30 percent of cases were recurrent.
There was a nonsignificant association between LP and infection and HCV. In our study, anti-HCV antibody was positive in 11 patients (33.3%) and negative in 19 patients (66.7%). Similarly, other studies report such an association, including investigations from Brazil and Slovenia.15,16 Conversely, Amer et al17 have found that approximately 70 percent of patients with LP were positive for HCV antibodies.
This variability in results may be attributed to several factors, including misdiagnosis of LP, the highly variable prevalence of HCV infection across the world, the prevalence of other etiologic factors of LP, and variability in the study designs.18
The efficacy of the combination of BCG-PSN and vitamin D was low relative to that of BCG-PSN treatment alone. We suggest that vitamin D may affect the mechanism of action of BCG-PSN at the immunological level during treatment of LP.
Conclusion
BCG-PSN alone is of value in the treatment of LP, while vitamin D in combination with BCG-PSN had no effect on cutaneous LP. However, based on the results of this study, a larger number of participants are needed for longer periods of treatment to detect possible unwanted side effects and the ideal dosing of intradermal BCG-PSN and booster doses is needed. Further studies should be done in areas of different social classes and endemic areas of tuberculosis to detect differences in response. Long-term follow up of patients is highly recommended to assess the long-term efficacy of BCG-PSN and the side effects. Finally, further studies of long durations of treatment with vitamin D are needed, and the dose of vitamin D has to be increased.
References
- Wee JS, Shirlaw PJ, Challacombe SJ, Setterfield JF. Efficacy of mycophenolate mofetil in severe mucocutaneous lichen planus: a retrospective review of 10 patients. Br J Dermatol. 2012;167(1):36–43.
- Parihar A, Sharma S, Bhattacharya SN, Singha UR. A clinicopathological study of cutaneous lichen planus. Journal of Dermatology & Dermatologic Surgery. 2015;19(1):21–26.
- Nahidi Y, TayyebiMeibodi N, Ghazvini K, et al. Association of classic lichen planus with human herpesvirus-7 infection. Int J Dermatol. 2017;56(1):49–53.
- Atas H, Cemil BC, Kurmus GI, et al. Assessment of systemic inflammation with neurophil-lymphocyte ratio in lichen planus. Postepy Dermatol Alergol. 2016;33(3):188–192.
- Thongprasom K. Dhanuthai K. Steroids in the treatment of lichen planus: a review. J Oral Sci. 2008;50(4):377–385.
- Zhou G, Fan MW, Liu JY. Regulation of BCG polysaccharide nucleic acid on Thl ?Th2 cytokines from peripheral blood mononuclear cells in oral lichen planus. Chin J Dent Res. 2004;7:5–10.
- Watkins RR, Yamshchikov AV, Lemonovich TL, Salata RA. The role of vitamin D deficiency in sepsis and potential therapeutic implications. J Infect. 2011;63(5):321–326.
- Uyl-de Groot CA, Vermorken JB, Hanna MG, Jr, et al. Immunotherapy with autologous tumor cell-BCG vaccine in patients with colon cancer: a prospective study of medical and economic benefits. Vaccine. 2005;23(17–18):2379–2387.
- Gupta SB, Chaudhari ND, Gupta A, et al. Lichen planus—an update. Int J Pharm Biomed Sci. 2013;4(2):59–65.
- Kurago, ZB. Etiology and pathogenesis of oral lichen planus: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(1):72–80.
- Varma RB, Valappila NJ, Pai A, et al. Oral lichen planus: Is vitamin D deficiency a predisposing factor? a case report. Int J Sci Stud. 2014;2(7):230–232.
- Xiong C, Li Q, Lin M, et al. The efficacy of topical intralesional BCG-PSN injection in the treatment of erosive oral lichen planus: a randomized controlled trial. J Oral Pathol Med. 2009;38(7):551–558.
- Metwalli MI, Marei AM, Toama MA, et al. Bacillus Calmette–Guerin polysaccharide nucleic acid extract versus triamcinolone acetonide intralesional injection in the treatment of oral lichen planus: a comparative study. Egypt J Dermatol Venerol. 2018;38(1): 1–11.
- Nasr MM, Ebrahim HM, Khattab FM, Marei AM. Bacillus Calmette–Guerin, polysaccharide nucleic acid in the treatment of cutaneous and oral lichen planus. Dermatol Ther. 2018;31(3):e12591.
- Maticic M, Poljak M, Lunder T, et al. Lichen planus and other cutaneous manifestations in chronic hepatitis C: pre and post-interferon-based treatment prevalence vary in a cohort of patients from low hepatitis C virus endemic area. J Eur Acad Dermatol Venereol. 2008;22(7):779–788.
- Stojanovic L, Lunder T, Poljak M, et al. Lack of evidence for hepatitis C virus infection in association with lichen planus. Int J Dermatol. 2008;47(12):1250–1256.
- Amer MA, El-Harras M, Attwa E, Raslan S. Lichen planus and hepatitis C virus prevalence and clinical presentation in Egypt. J Eur Acad Dermatol Venereol. 2007; 21(9):1259–1260.
- Lodi G, Pellicano R, Carrozzo M. Hepatitis C virus infection and lichen planus: a systematic review with meta-analysis. Oral Dis. 2010;16(7):601–612.

