 J Clin Aesthet Dermatol. 2022;15(2):23–25.
J Clin Aesthet Dermatol. 2022;15(2):23–25.
by Alessio Gambardella, MD; Gaetano Licata, MD; Alina De Rosa, MD; Giulia Calabrese, MD; Roberto Alfano, MD and Giuseppe Argenziano, MD
Drs. Gambardella, Licata, De Rosa, Calabrese, Argenziano are with the Dermatology Unit, Department of Mental and Physical Health and Preventive Medicine at the University of Campania Luigi Vanvitelli in Naples, Italy. Dr. Alfano is with the Department of Anesthesiology, Surgery and Emergency at the University of Campania Luigi Vanvitelli in Naples, Italy.
ABSTRACT: Introduction. Recently it has been reported that apremilast might promote melanogenesis and it would therefore not be safe to use this drug in patients with psoriasis who have a history of melanoma.
Methods. From January 2017 to December 2020, we retrospectively identified, within a cohort of 635 patients in follow-up care for melanoma, 16 cases of patients with psoriasis treated with apremilast and history of melanoma Patients were monitored at our unit for a mean duration of 36 months of follow up.
Results. The use of apremilast, in our case series was, thus, effective in managing psoriasis without causing recurrence of previous melanoma or any new suspicious lesions which would need removal.
Discussion. It has been speculated that apremilast might not be safe in patients who have a history of melanoma as it would be involved in the stimulation of melanogenesis and consequent possibility of recurrence of previous melanoma.
Conclusion. Our data show that none of the patients treated with apremilast developed recurrence of melanoma at 36 months of follow-up. Further studies are necessary to confirm the safety of apremilast in a larger number of patients with concurrent malignancies, specifically melanoma, and for a longer follow-up period.
Keywords: Apremilast, melanoma, psoriasis
Psoriasis is a chronic, refractory, inflammatory disease of the skin and joints affecting 2 to 3 percent of the Caucasian population.1 Data found in the literature have shown that patients with chronic inflammatory diseases might have an increased risk of cancer due to impaired immunosurveillance and immunosuppressive pharmacological agents.2 Patients with psoriasis have a higher risk of non-melanoma skin cancer; especially, squamous cell carcinomas often result from previous treatments with ciclosporin and photochemotherapy (PUVA).3-4 However, data concerning the association of melanocytic nevi and melanoma risk with psoriasis are lacking and controversial. One Swedish cohort study reported that the risk for melanoma in patients with psoriasis may be lower than in individuals without psoriasis.5 Moreover, Balato et al6 suggest a protective role of psoriasis against melanocytic nevi and melanoma. At the same time, treating patients with psoriasis and a history of past or concomitant malignancy is a challenge. In fact, in these cases it is not possible to use either traditional agents or biological drugs. In contrast to the majority of the available systemic and biological antipsoriatic drugs, apremilast is not contraindicated in the case of previous or concomitant malignancy.7 Recently it has been reported, however, that apremilast might promote melanogenesis and it would therefore not be safe to use this drug in patients with psoriasis who have a history of melanoma.8 For this reason, we report our clinical experience of patients with previous melanoma treated and monitored at our unit for a mean duration of 36 months of follow-up who had a moderate to severe psoriasis which had been treated with apremilast. The purpose of our case series was to evaluate the safety and tolerability of apremilast in this group of patients with psoriasis and history of melanoma.
Methods
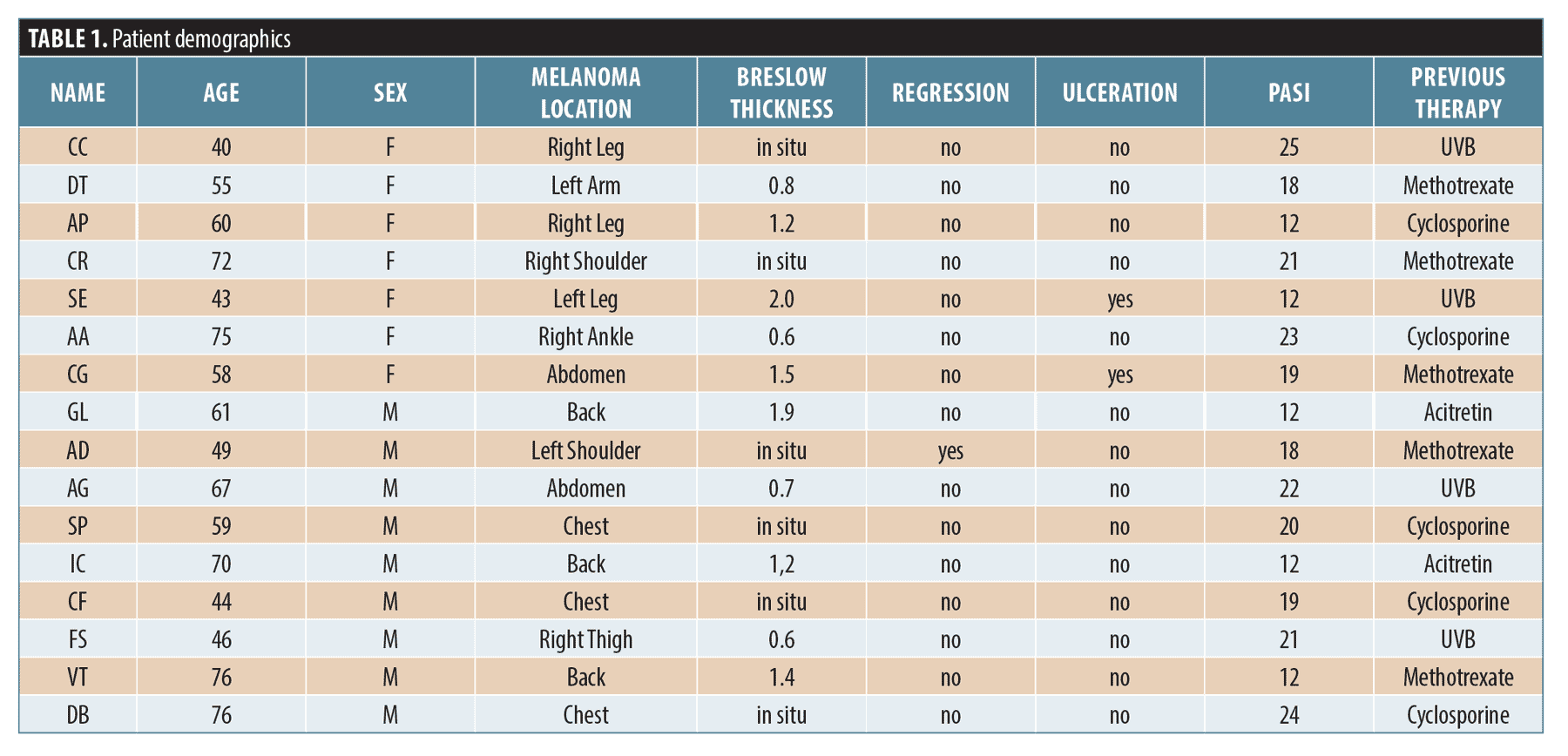
From January 2017 to December 2020, we retrospectively identified, from our research database, within a cohort of 635 patients in follow-up care for melanoma, 16 cases of patients (9 males and 7 females) aged between 40 and 72 years, with psoriasis treated with apremilast and history of melanoma. The histopathologic parameters recorded on the database were: Breslow thickness, Clark level, degree of regression, presence of ulceration. We also collected data on age at diagnosis of psoriasis, Psoriasis Area and Severity Index score (PASI), Body Surface Area (BSA), previous and current therapies. Psoriasis severity was classified as moderate to severe. Furthermore, it was also assessed how long the patients had been on apremilast treatment and any reported side effects. Patients were evaluated every 4 to 6 months: clinical examination, total body photographic documentation were performed in all the cases. Informed consent was obtained from all participants.
Results
Among these 16 patients, 10 had had invasive melanoma ranging in thickness from 0.5 mm to 2.0 mm sec Breslow; the remaining six patients had a history of melanoma in situ. All patients selected had a history of psoriasis vulgaris for more than 20 years (20–25 years) treated with topical agents and/or conventional systemic treatment, and now treated with apremilast. Our data showed that at the time of melanoma diagnosis: 75 percent of patients had severe psoriasis with PASI between 18–25 and BSA 18; the remaining 25 percent of patients had psoriasis with PASI 12 and BSA 7 (all patients’ details are listed in Table 1). Considering the clinical history of previous malignancy, all patients were treated with apremilast at a dosage of 30mg twice daily after an initial titration on Day 1 until Day 5 for a mean period of 24 months. All patients showed noticeable improvement in psoriasis after six months with an average PASI of 2 and BSA 1. Side effects reported were nausea and diarrhoea during the first few weeks of treatment. On clinical examination and according to diagnostic tests (ultrasound and/or CT), no patients showed any local or systemic recurrence of previous melanoma after 36 months of follow up. Furthermore, no new multiple hyperpigmented asymptomatic macules or new melanocytic nevi or lentigines in areas previously affected by psoriasis plaques were detected. The use of apremilast, in our case series was, thus, effective in managing psoriasis skin lesions without causing recurrence of previous melanoma or any new suspicious lesions which would need removal.
Discussion
Apremilast is a small oral molecule that selectively inhibits phosphodiesterase 4 (PDE-4) approved for the treatment of psoriasis, psoriatic arthritis and Behçet’s syndrome.9 The PDE-4 inhibitor produces the elevation of intracellular cyclin adenosine monophosphate (cAMP), causing down-regulation of pro-inflammatory cytokines/chemokines, such as tumour necrosis factor, interleukin 23-12, interleukin 17 and interferon gamma. It also causes decrease of T helper 1 and Th17 response, and, as is also observed, upregulation of anti-inflammatory cytokines such as IL-10.10 Recently, apremilast has been reported as causing post-inflammatory lentiginosis and cutaneous hyperpigmentation after treatment for psoriasis since apremilast, by increasing the level of cAMP, would lead melanocytes to produce pigment.11-12 Therefore, it has been speculated that this drug may not be safe in patients who have a history of melanoma as it would be involved in the stimulation of melanogenesis and consequent possibility of recurrence of previous melanoma.8 Furthermore, cAMP and PDE-4 have also been studied in melanoma and other malignancies, and it has been hypothesized that they may be involved in tumor progression and metastatic invasion.13 It has also been postulated that the use of apremilast could impair cancer immunosurveillance and lead to a recurrence of melanoma.8 The development of lentigines in areas previously affected by psoriasis is a rare event; it has been reported after phototherapy or after treatment with anti TNF-alpha or anti-interleukin 12/23. The pathophysiology of lentigines in psoriatic lesions is poorly understood.14 With respect to this, it has been previously reported that the cytokines involved in the pathogenesis of psoriasis inhibit tyrosinase activity with reduction of melanogenesis. It was therefore assumed that the rapid decrease in proinflammatory cytokine caused by the antipsoriatic treatments could lead to melanogenesis signalling pathways.11 However, a few cases regarding cutaneous hyperpigmentation induced by apremilast or other biological drugs have been reported.11,12,15,16 However, the occurrence of lentigines after treatment with apremilast was not reported in any of the 4000 patients enrolled in the Phase II or Phase III studies.17,18 Therefore, we can assert that apremilast is not involved in the melanogenesis process. In addition, only one possible case of recurrence of melanoma after starting apremilast for psoriasis has been reported by Salopek,8 who described the case of a 32-year-old man with a history of two previous invasive melanoma. The first melanoma, localized on the right occiput with a thickness of 1.53 mm sec Breslow, was followed by nodal recurrence and treated with complete lymph nodes dissection. The second melanoma localized on the right upper chest had a thickness of 0.9 sec Breslow. The author also reported that the patient had severe psoriasis and, that after being disease-free from his melanoma for more than three years, he had begun therapy with apremilast, and that after only four months of therapy with apremilast the patient had developed a recurrence of melanoma. Moreover, the author hypothesized that the use of apremilast in this patient resulted in impaired cancer immunosurveillance which led to a recurrence of his melanoma. In our opinion, taking into account the patient’s clinical history and the limited period of use of apremilast, four months, we can exclude that apremilast may have a role in causing recurrence of melanoma. Several data have shown, rather, that apremilast is a safe option in treating psoriasis in patients with previous or concomitant malignancies. Recently, Siciliano et al19 reported the first evidence of the use of apremilast in immunotherapy-induced psoriasis in a melanoma patient, suggesting not only that apremilast is a safe option in a metastatic patient, but also that apremilast does not interfere with pembrolizumab anti-cancer activity. In our present study, all patients treated with apremilast showed a significant improvement in their psoriasis. None of them showed local or systemic recurrence of previous melanoma after 36 months of follow-up care. These results indicate the safety, the efficacy and the tolerability of apremilast in patients with psoriasis and history of cancer.
Conclusion
Evidence on the safety of small molecules in the context of patients with psoriasis and concomitant malignancies is generally lacking due to exclusion of patients with cancer from clinical trials.20 However, considering the mechanism of action of apremilast, its use with these patients with a history of cancer could be considered safe. The evidence supporting a possible role of apremilast in stimulation of melanogenesis is scarce. There is, however, limited data on the incidence of malignancies, including melanoma, in psoriatic patients. Several studies have reported that psoriatic patients have fewer melanocytic nevi than control subjects suggesting a protective role of the immune pathogenic background of psoriasis against development of melanocytic lesions. Our data show that none of the patients treated with apremilast developed recurrence of melanoma at 36 months of follow-up. Further studies are, however, necessary to confirm the safety of apremilast in a larger number of patients with concurrent malignancies, specifically melanoma, and for a longer follow-up period.
References
- Vujic I, Herman R, Sanlorenzo M, Posch C, Monshi B, Rappersberger K, Richter L. Apremilast in psoriasis – a prospective real-world study. J Eur Acad Dermatol Venereol. 2018 Feb;32(2):254-259.
- Pouplard C, Brenaut E, Horreau C, Barnetche T, Misery L, Richard MA, Aractingi S, Aubin F, Cribier B, Joly P, Jullien D, Le Maître M, Ortonne JP, Paul C. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013 Aug;27 Suppl 3:36-46.
- Rademaker M, Rubel DM, Agnew K, Andrews M, Armour KS, Baker C, Foley P, Gebauer K, Goh MS, Gupta M, Marshman G, Prince HM, Sullivan J. Psoriasis and cancer. An Australian/New Zealand narrative. Australas J Dermatol. 2019 Feb;60(1):12-18.
- Stern RS, Nichols KT, Väkevä LH. Malignant melanoma in patients treated for psoriasis with methoxsalen (psoralen) and ultraviolet A radiation (PUVA). The PUVA Follow-Up Study. N Engl J Med. 1997 Apr 10;336(15):1041-5.
- Boffetta P, Gridley G, Lindelöf B. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden. J Invest Dermatol. 2001 Dec;117(6):1531-7.
- Balato N, Di Costanzo L, Balato A, Patruno C, Scalvenzi M, Ayala F. Psoriasis and melanocytic naevi: does the first confer a protective role against melanocyte progression to naevi? Br J Dermatol. 2011 Jun;164(6):1262-70.
- Kahn JS, Casseres RG, Her MJ, Dumont N, Gottlieb AB, Rosmarin D. Treatment of Psoriasis With Biologics and Apremilast in Patients With a History of Malignancy: A Retrospective Chart Review. J Drugs Dermatol. 2019 Apr 1;18(4).
- Salopek TG. Recurrence of Melanoma after Starting Apremilast for Psoriasis. Case Rep Dermatol. 2017 Aug 3;9(2):108-111.
- Keating GM. Apremilast: A Review in Psoriasis and Psoriatic Arthritis. Drugs. 2017 Mar;77(4):459-472.
- Schafer PH, Parton A, Capone L, Cedzik D, Brady H, Evans JF, Man HW, Muller GW, Stirling DI, Chopra R. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014 Sep;26(9):2016-29.
- Vazquez B, Gonzalez V, Molina I, Montesinos E, Ramon MD, Monteagudo C. Multiple lentigines arising on resolving psoriatic plaques after treatment with apremilast. Clin Exp Dermatol. 2019 Jan;44(1):66-67.
- Sfecci A, Khemis A, Lacour JP, Montaudié H, Passeron T. Appearance of lentigines in psoriasis patients treated with apremilast. J Am Acad Dermatol. 2016 Dec;75(6):1251-1252.
- Delyon J, Servy A, Laugier F, André J, Ortonne N, Battistella M, Mourah S, Bensussan A, Lebbé C, Dumaz N. PDE4D promotes FAK-mediated cell invasion in BRAF-mutated melanoma. Oncogene. 2017 Jun 8;36(23):3252-3262.
- Wang CQF, Akalu YT, Suarez-Farinas M, Gonzalez J, Mitsui H, Lowes MA, Orlow SJ, Manga P, Krueger JG. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013 Dec;133(12):2741-2752.
- Di Cesare A, Pescitelli L, Ricceri F, Lazzeri L, Prignano F. Cutaneous hyperpigmentation induced by apremilast. Int J Dermatol. 2018 Apr;57(4):473-474.
- McCarthy S, Heffron CCBB, Murphy M. Lentigines within fixed drug eruption: reply to ‘Multiple lentigines arising on resolving psoriatic plaques after treatment with apremilast’. Clin Exp Dermatol. 2019 Apr;44(3):358-359.
- Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C, Crowley J, Hu C, Stevens RM, Shah K, Day RM, Girolomoni G, Gottlieb AB. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015 Dec;173(6):1387-99.
- Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, Hu C, Stevens RM, Day RM, Gordon KB, Korman NJ, Griffiths CE. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015 Jul;73(1):37-49.
- Siciliano MA, Dastoli S, d’Apolito M, Staropoli N, Tassone P, Tagliaferri P, Barbieri V. Pembrolizumab-Induced Psoriasis in Metastatic Melanoma: Activity and Safety of Apremilast, a Case Report. Front Oncol. 2020 Oct 14;10:579445.
- Apalla Z, Psarakis E, Lallas A, Koukouthaki A, Fassas A, Smaragdi M. Psoriasis in Patients With Active Lung Cancer: Is Apremilast a Safe Option? Dermatol Pract Concept. 2019 Oct 31;9(4):300-301.

